Analysis of pharmaceuticals in water by automated solid-phase extraction
By Biotage
The purpose of this application note is to outline an automated extraction method utilizing the Atlantic® HLB-M SPE Disk and Biotage automated and manual SPE solutions for the extraction of pharmaceuticals in water. The first section will highlight the use
of the Biotage® Horizon 5000 fully automated extraction system and the method used for this application. Additionally, there will be an Application Modification section that will highlight the use of the Biotage® Horizon 4790 (with data and discussion) and Biotage® VacMaster™ Disk for this application.
Introduction
The analytical challenge of measuring emerging contaminants in the environment has been a major research focus of scientists for the last 20 years. Water quality is a critical issue especially for sustainable socioeconomic development. Anthropogenic activities are one of the main causes for water quality damage and, consequently, social concern calls for quality control action. Even after water treatment, it has been demonstrated in many studies that organic contaminants escape conventional wastewater treatment processes, and they end up in aquatic systems.
Pharmaceuticals and personal care products are an important group of contaminants that have been targeted, especially in the last decades. For example, EPA Method 16941, published in December 2007, is a guiding and screening method for those scientists analyzing pharmaceuticals in environmental samples. The standard EPA protocol uses solid phase extraction (SPE) for water samples followed by the analysis of extracts by tandem mass spectrometry using a single transition for each compound, with retention time guidelines for identification.
Contaminants are usually present in the environmental samples at very low concentration levels (ng/L) and, for this reason, solid phase extraction techniques are often used to isolate and pre-concentrate the organic compounds of interest. This has led to the development of a method for low concentration level analysis of pharmaceuticals in drinking water samples. The implementation for this method consists of the analysis of 20 analytes which are some of the most common contaminants found in the environment.
Figure 1: Biotage® Horizon 5000.
Figure 2: TurboVap® .
Instrumentation
» Biotage Instruments
» Biotage® Horizon 5000 Automated Extraction System
» Atlantic® HLB-M SPE Disk
» TurboVap® Automated Solvent Evaporation System
» Agilent
» 1200 HPLC and 6220 LC-TOF-MS
» Zorbax Eclipse XDB-C8 Cartridge
Biotage® Horizon 5000 method summary
- Purge the Biotage® Horizon 5000 system using the generic method shown in Table
- Obtain water samples. No additional additives or filtration needed.
- Spike any control or matrix spike samples using a spiking mixture containing the 20 pharmaceutical compounds at 0.5 µg/L.
- Attach the sample bottle to the water inlet valve and then place it onto the extractor.
- Extract water samples using the Biotage® Horizon 5000 system with the method shown in Table 2, resulting in approximately 40 mL of extract.
- Transfer the extracts to a 45 °C water bath and concentrate with a gentle stream of nitrogen to near dryness.
- Reconstitute the dried sample in a acetonitrile: deionized water (1:9, v/v) solution.
- Analyze the extracts via LC-TOF-MS utilizing the parameters outlined in the sample analysis section below.
|
Step |
Select Solvent |
Volume (mL) |
Purge (s) |
Vacuum |
Saturate (s) |
Soak (s) |
Drain/Elute (s) |
Sample Delay (s) |
|---|---|---|---|---|---|---|---|---|
|
Condition SPE Disk |
Reagent Water |
20 |
60 |
2 |
1 |
0 |
30 |
|
|
Condition SPE Disk |
Methanol |
20 |
60 |
2 |
1 |
0 |
30 |
|
|
Wash Sample Container |
Reagent Water |
20 |
15 |
2 |
1 |
0 |
30 |
|
|
Elute Sample Container |
Methanol |
20 |
15 |
6 |
1 |
0 |
30 |
|
Step |
Select Solvent |
Volume (mL) |
Purge (s) |
Vacuum |
Saturate (s) |
Soak (s) |
Drain/ Elute (s) |
Sample Delay (s) |
|
Condition SPE Disk |
Acetone |
11 |
60 |
2 |
1 |
30 |
30 |
|
|
Condition SPE Disk |
Acetone |
11 |
60 |
2 |
1 |
30 |
30 |
|
|
Condition SPE Disk |
Reagent water |
15 |
60 |
2 |
1 |
10 |
4 |
|
|
Condition SPE Disk |
Reagent water |
15 |
60 |
2 |
1 |
10 |
4 |
|
|
Load Sample |
|
|
|
2 |
|
|
|
45 |
|
Air Dry Disk |
|
|
|
6 |
|
|
60 |
|
|
Elute Sample Container |
Acetone |
8 |
15 |
2 |
1 |
180 |
40 |
|
|
Elute Sample Container |
Dichloromethane |
8 |
15 |
2 |
1 |
180 |
40 |
|
|
Elute Sample Container |
Dichloromethane |
8 |
15 |
2 |
1 |
60 |
40 |
|
|
Elute Sample Container |
Dichloromethane |
8 |
15 |
6 |
1 |
60 |
120 |
|
Sample analysis
The separation of the analytes was carried out using an HPLC system equipped with a reversed phase C8 analytical cartridge of 150 mm x 4.6 mm and 5 µm particle size. Cartridge temperature was maintained at 25 °C. The injected sample volume was 50 µL. Mobile phases A and B were acetonitrile and water with 0.1% formic acid, respectively. The optimized chromatographic method held the initial mobile phase composition (10% A) constant for 5 min, followed by a linear gradient to 100% A after 30 min. The flowrate used was 0.6 mL/min. A 10 min post-run time was used after each analysis. This HPLC system was connected to a time-of-flight mass spectrometer Agilent 6220 MSD TOF equipped with a dual electrospray source, operating in positive ion mode, using the following operation parameters: capillary voltage: 4000 V; nebulizer pressure: 45 psig; drying gas: 9 L/min; gas temperature: 300 °C; fragmentor voltage: 190 V; skimmer voltage: 60 V; octopole RF: 250 V.
Acknowledgements
Biotage would like to thank E. Michael Thurman and Imma Ferrer of the Center for Environmental Mass Spectrometry at the University of Colorado in Boulder for their assistance in developing this method.
References
- EPA Method 1694: Pharmaceuticals and personal care products in water, soil, sediment, and biosolids by HPLC/ MS/MS, December 2007, EPA-821-R-08-002.
- Ferrer, I., Zweingenbaum, J.A., Thurman, E.M., J. Chromatogr. A, 1217 (2010) 5674-5686
Method modifications
Biotage® Horizon 4790 method summary
- Purge the Biotage® Horizon 4790 system using the generic method shown in Table 3.
- Obtain water samples. No additional additives or filtration needed.
- Spike any control or matrix spike samples with spiking mixture containing the 20 pharmaceutical compounds at 0.5 µg/L.
- Cover the sample bottle with the EZ foil and cap and place onto the Horizon 4790 turning the sample bottle ¾ of the way to move most of the foil out of the way.
- Extract the water samples using the Horizon 4790 system with the method shown in Table 4.
- Transfer the extracts to a 45ºC water bath and concentrate with a gentle stream of nitrogen to near dryness.
- reconstitute the dried sample with acetonitrile / deionized water (1:9, v/v) solution.
- Analyze the extracts via LC-TOF-MS utilizing the parameters outlined in the sample analysis section above.
|
Step |
Solvent |
Soak Time (s) |
Dry Time (s) |
|
Prewet 1 |
Reagent Water |
0 |
15 |
|
Prewet 2 |
Methanol |
0 |
15 |
|
Wash 1 |
Reagent Water |
0 |
15 |
|
Rinse 1 |
Methanol |
0 |
15 |
|
Step |
Solvent |
Soak Time (s) |
Dry Time (s) |
|
Prewet 1 |
Acetone |
30 |
15 |
|
Prewet 2 |
Acetone |
30 |
15 |
|
Prewet 3 |
Reagent Water |
10 |
2 |
|
Prewet 4 |
Reagent Water |
10 |
2 |
|
Sample Process |
|
|
|
|
Air Dry |
|
|
30 |
|
Rinse 1 |
Acetone |
180 |
20 |
|
Rinse 2 |
Methylene Chloride |
180 |
20 |
|
Rinse 3 |
Methylene Chloride |
60 |
20 |
|
Rinse 4 |
Methylene Chloride |
60 |
20 |
|
Rinse 5 |
Methylene Chloride |
60 |
60 |
Biotage® Horizon 4790 results and discussion
The extracts were analysed by LC-TOF-MS. The compounds were chromatographically separated and detected by accurate mass measurements. The recoveries and relative standard deviations (RSD) for the selected pharmaceuticals are within EPA’s 1694 method criteria for precision and recovery. The results for three replicates are presented in Table 5 and a sample chromatogram for the compounds is given in Figure below.
|
Analyte |
Use |
Average Recovery (%) |
RSD (%) |
|
Acetaminophen |
Non-steroidal anti inflammatory |
65 |
8 |
|
Albuterol |
Bronchodilator |
79 |
5 |
|
Atenolol |
Antihypertensive |
86 |
3 |
|
Caffeine |
Cardiac and respiratory stimulant |
66 |
5 |
|
Carbamazepine |
Anticonvulsant/Antidepressant |
101 |
2 |
|
Cotinine |
Antidepressant |
86 |
5 |
|
DEET |
Mosquito repellant |
89 |
6 |
|
Dehydronifedipine |
Antihypertensive |
91 |
5 |
|
Diclofenac |
Anti-inflammatory |
88 |
9 |
|
Diltiazem |
Antihypertensive |
71 |
8 |
|
Diphenhydramine |
Antihistamine |
76 |
5 |
|
Gemfibrozil |
Non-steroidal anti inflammatory |
101 |
2 |
|
Ibuprofen |
Non-steroidal anti inflammatory |
108 |
5 |
|
Lamotrigine |
Antidepressant |
95 |
3 |
|
Metoprolol |
Antihypertensive |
73 |
5 |
|
Naproxen |
Non-steroidal anti inflammatory |
110 |
7 |
|
Sulfadimethoxine |
Antibiotic |
85 |
5 |
|
Sulfamethoxazole |
Antibiotic |
46 |
8 |
|
Triclocarban |
Antiseptic |
65 |
5 |
|
Trimethoprim |
Antibacterial |
83 |
3 |
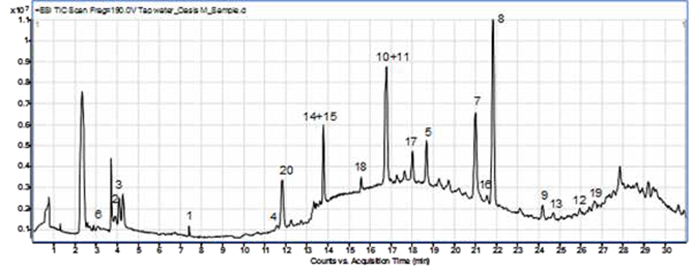
Figure 3: LC-TOF-MS chromatogram of a spiked tap water sample after extraction with the Biotage® Horizon 4790.
Conclusion
The results demonstrated that the Biotage® Horizon 4790 using Atlantic® HLB-M disks can effectively extract pharmaceutical compounds from 1 L water samples in approximately 40 minutes. This system allows you to use the original sample bottle which will be rinsed with all of the extraction solvents before the elution step. This rinsing step ensures that all the compounds are rinsed off the glass and retained on the disk.
Biotage® VacMaster™ Disk method summary
- Repeat the following steps for each active Biotage® VacMaster™ Disk station.
- Setup the VacMaster Disk manifolds ensuring all waste lines and vacuum lines are attached. Set the vacuum pump to -24”Hg.
- Prepare the disk holder assembly (47 mm): ensure the support screen is flat in the centre of the disk holder. Place the Atlantic® HLB-M Disk on top of the support screen with the ripples of the disk on top and add any prefilters on top of the disk. Place the disk holder assembly on the VacMaster Disk manifold ensuring there is a tight seal with the luer fitting.
- If using the multifunnel, place onto the disk holder assembly. If not using the multifunnel, omit those directions throughout the method.
- Condition the SPE Disk.
- Guide for each conditioning step in table 6 below:
- Measure the appropriate VOLUME of SOLVENT into a graduated cylinder and pour into the disk holder assembly.
- Using a Nalgene Wash Bottle (phthalate free), rinse the multifunnel and disk holder in a circle for about 3 seconds using the same SOLVENT (approximately 5 additional mL).
- SATURATE the disk for the time indicated (in SECONDS). (Saturate means: quickly turn the knob to the appropriate waste destination and back to the “OFF” position. This brings the solvent into the disk media bed).
- SOAK the disk for the time indicated (in SECONDS).
- DRAIN to the appropriate waste destination for the time indicated (in SECONDS). Switch to the “OFF” position.
Solvent
Volume (mL)
Saturate (sec)
Soak (sec)
Waste Destination
Drain (sec)
Acetone
11
1
30
Organic
30
Acetone
11
1
30
Organic
30
Reagent Water
15
1
10
Organic
4
Reagent Water
15
1
10
Aqueous
4
- Guide for each conditioning step in table 6 below:
- Load the Sample
- For multifunnel: quickly and efficiently angle the bottle to rest on the multifunnel upside-down.
- For no multifunnel: pour a portion of the sample into the disk holder.
- Adjust the vacuum between -10”Hg and -15”Hg for sample load (please note, if the sample is flowing too slowly, the vacuum can be increased). Drain the sample to “AQUEOUS” waste. Continue to pour the sample into the disk holder ensuring the disk does not go dry or overflow for the duration of sample load.
- Air Dry the SPE Disk:
- Return the vacuum to -24”Hg and continue to air dry the SPE disk to “AQUEOUS” waste for an additional 60 SECONDS. Switch to the “OFF” position.
- Remove the sample bottle from the multifunnel if it was used.
- 8. Elute the SPE Disk: (Please note: the elution solvent will go into the collection flask inside the chamber, not to waste containers).
- Place a clean 125 mL 24/40 tapered Erlenmeyer flask or 40 mL VOA vial using the VOA vial insert into the Biotage® VacMaster™ Disk collection chamber. Place the cover
on the chamber. Remove the disk holder assembly and place the disk holder assembly into the luer fitting on top of the collection chamber. Attach the luer fitting of the collection chamber assembly onto the manifold. - Guide for each elution step in table 7 below:
- Measure the appropriate VOLUME of SOLVENT into a graduated cylinder, pour into the sample bottle, and swirl around. Pour the solvent in the sample bottle into the disk holder assembly.
- Using a Nalgene Wash Bottle (phthalate free), rinse the multifunnel and disk holder in a circle for about 3 seconds using the same SOLVENT (approximately 5 additional mL).
- SATURATE the disk for the time indicated (in SECONDS) to “ORGANIC”.
- SOAK the disk for the time indicated (in SECONDS).
- DRAIN to “ORGANIC” for the time indicated (in SECONDS). Switch to the “OFF” position.
- Remove the chamber lid to release the vacuum from inside the chamber.
- Place a clean 125 mL 24/40 tapered Erlenmeyer flask or 40 mL VOA vial using the VOA vial insert into the Biotage® VacMaster™ Disk collection chamber. Place the cover
|
Solvent |
Volume (mL) |
Saturate (sec) |
Soak (sec) |
Waste Destination |
Elute (sec) |
|---|---|---|---|---|---|
|
Acetone |
8 |
1 |
180 |
Organic |
40 |
|
Methylene |
8 |
1 |
180 |
Organic |
40 |
|
Methylene Chloride |
8 |
1 |
60 |
Organic |
40 |
|
Methylene |
8 |
1 |
60 |
Organic |
40 |
Literature number: AN080-HOR

