Feb 10, 2023 3:47:38 PM
Why is reversed-phase flash chromatography use increasing?
By Bob Bickler

Reversed-phase flash chromatography usage is increasing rapidly. In fact, over the past 10 or so years, reversed-phase flash chromatography use has increased a dramatic 650%! This is amazing growth despite the fact that reversed-phase flash columns are considerably more expensive than silica columns and you need to evaporate water from your fractions. So, what’s driving this change in chemists’ modus operandi?
In this post, I will explain why chemists are increasingly using reversed-phase flash chromatography for routine, intermediate, and final compound purification and provide and example as well.
Historically, synthetic chemists have viewed reversed-phase chromatography primarily as an analytical tool (HPLC) to determine if a reaction has created a desired product (and how much) and determine the by-products and impurities. Likewise, these same chemists typically use normal-phase flash chromatography when the crude mix requires purification.
While this approach has worked for decades, just because a separation is possible by reversed-phase HPLC does not mean normal-phase flash chromatography will be successful (there is no direct way to transfer reversed-phase methods to normal-phase).
For chemists, the ultimate goal is target compound isolation with enough purity and yield to move to the next synthesis step. So, what do you do when normal-phase does not purify your product? Well, why not try reversed-phase flash chromatography? Apparently, chemists across the globe are using this technique in more and more.
Some reasons for the migration to reversed-phase include...
- Synthesis of increasingly complex reaction mixtures (hard to remove by-products)
- Synthesis of increasingly polar compounds (increasing polar solvent use)
- Focus on reducing environmental impact/green chemistry
- Reduced organic solvent use
- Column reusability (less landfill impact and lower costs)
- Reduced consumable expense (solvent and columns)
- Cost savings
As an example, let’s look at one of these "hard-to-remove-by-products" situations with a real reaction mixture, Figure 1.
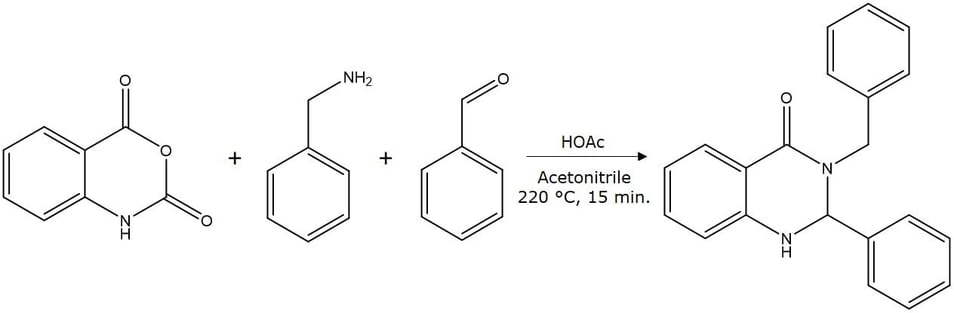
Figure 1. Synthesis of 3-benzyl-2-phenyl-2,3-dihydro-4(1H)-quinazolinone, MW 314.
To get an idea of the reaction mixture’s composition I evaluated the crude mix by silica TLC (40% ethyl acetate in hexanes), Figure 2. From previous experience with this reaction, I know my product fluoresces (purple/blue on the TLC plate) as does the closely eluting by-product. I used these data to create my normal-phase flash chromatography method.

Figure 2. Reaction mixture TLC in 40% EtOAc/hexanes. Reaction product is the dark, large blue/purple spot with a closely-eluting by-product trailing it.
I also analyzed the reaction mixture with a Biotage® Isolera Dalton 2000 that confirmed my product’s presence (+m/z 314.8 and –m/z of 312.8). Some of the other major mass responses were +m/z 235.4 and +m/z 226.8.
For normal-phase flash purification, I used a 5-gram high performance silica column (Biotage® Sfär HC) and a TLC-based hexane/ethyl acetate gradient (10-80%). I loaded 30 mg of my crude onto the column via dry loading with a silica Samplet® cartridge.
This chromatography shows that the trailing by-product on TLC has the +m/z 226.8 and co-elutes with my product, Figure 3. The early eluting by-product on TLC has the +m/z 235.4 mass and is well resolved from my product. Other normal-phase gradients could not separate the +m/z 226.8 compound either so I thought I would try reversed-phase.
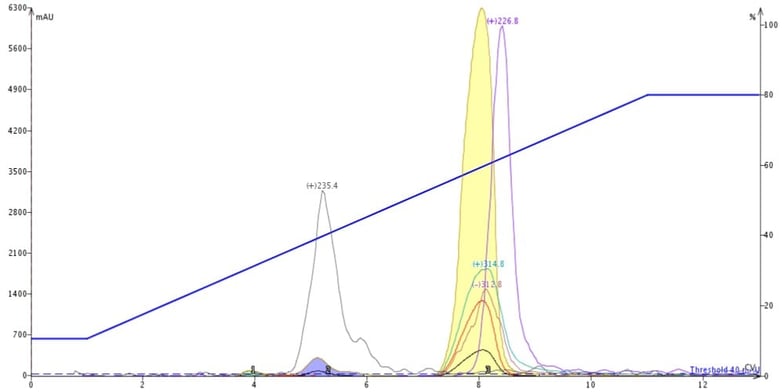
Figure 3. Reaction mixture purification using normal-phase flash chromatography shows the presence of a closely-eluting impurity with a +m/z of 226.8 at the tail of the product (yellow peak).
Since reversed-phase chromatography separates compounds using a different mechanism (solute partitioning) it usually elutes compounds in reversed-order compared to normal-phase (hence reversed-phase). Because of this fundamental difference I thought reversed-phase flash chromatography could be successful at removing the trailing impurity from my product.
For reversed-phase, I used a 6-gram high-performance Biotage® Sfär C18 column with a water/acetonitrile gradient (50-80%) and dry-loaded 30 mg of crude reaction mixture with a Samplet cartridge. I was very pleased to see that the C18 column removed both the trailing by-product (+m/z 226.8) the other major impurity (+m/z 235.4) from my product, Figure 4.
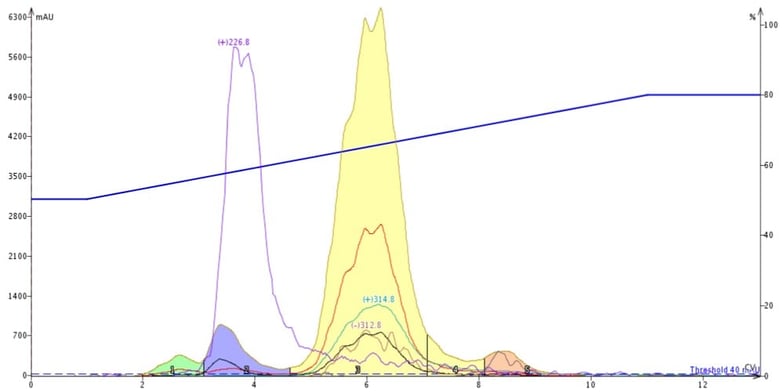
Figure 4. Reversed-phase flash chromatography successfully removed the major impurities from my target product (yellow peak).
Though I cannot promise reversed-phase flash chromatography will always out-perform normal-phase, I believe that if you have a tough-to-purify mixture not purifiable using normal-phase flash chromatography, you owe it to yourself to try this alternative strategy. You may be pleasantly surprised with the results as I was with this purification.
If you are interested in learning more about flash chromatography, please follow the link below!
Published: Feb 10, 2023 3:47:38 PM


