Apr 17, 2023 10:08:25 AM
When should I use C18 rather than silica for flash chromatography?
By Bob Bickler

Flash chromatography is nearly ubiquitous within organic and medicinal chemistry labs. Most chemists, when the need arises to purify a reaction mixture, will use a silica column (aka normal-phase) for flash chromatography. This purification technique utilizes a column filled with silica and lipophilic solvents such as hexane, heptane, dichloromethane, and ethyl acetate. For polar and/or basic compounds, chemists will add methanol and a strong base (for basic compounds) to try the get their product to separate and elute.While this purification strategy has provided acceptable results (it is the most commonly used flash chromatography technique), it is not necessarily the most efficient purification strategy or provide the best results. Sometimes, normal-phase flash chromatography does not work at all, especially with polar reaction solvents and polar reaction products!
It has been my experience that C18 (reversed-phase) flash chromatography can solve many of the challenges of using silica column chromatography. Not only will reversed-phase provide separations of polar reaction products, but also of non-polar as well.
How do you, as the chemist, decide between silica and C18 flash chromatography?
For me, I start with reaction mixture solubility. In most cases, if the reaction is performed in a polar solvent (DMF, DMSO, MeCN, etc.) or is polar solvent soluble, I automatically opt for reversed-phase because the reaction products are likely polar and there is far less pre-purification reaction work-up. If you are unsure, Table 1 provides a listing of sample/reaction solvents and their suggested purification mode.
Table 1. Reaction/sample solvent - purification mode table.
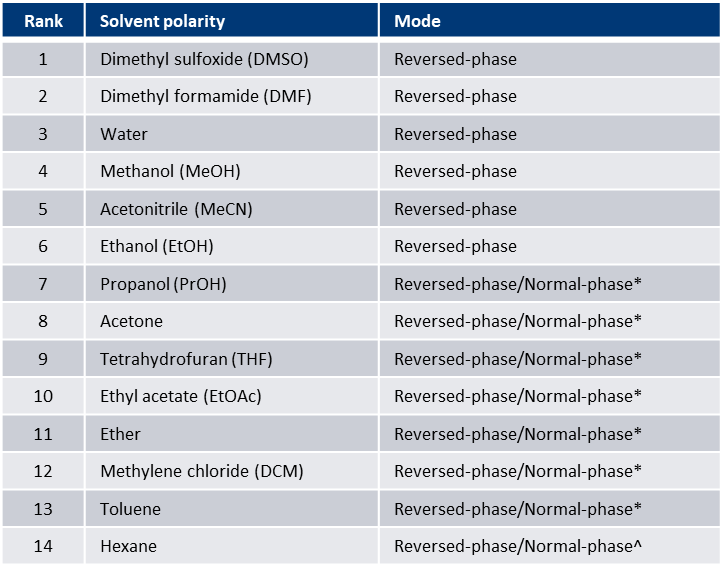 * Dry loading is best for these solvents
* Dry loading is best for these solvents
^ Dry loading is best for reversed-phase loading
A good example of this strategy is the purification of this synthetic quinazolinone, Figure 1.
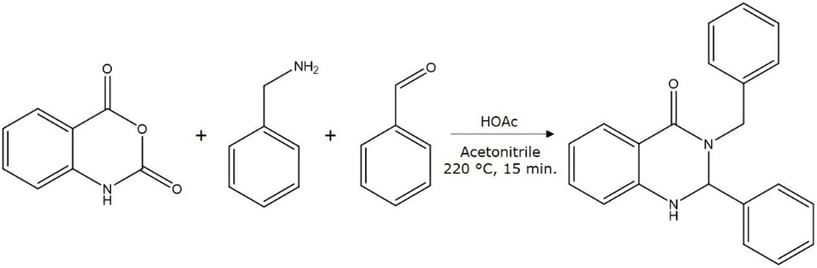
Figure 1. Quinazolinone reaction.
The reaction mixture was analyzed using TLC with hexane/ethyl acetate (7:3) to see if normal-phase flash chromatography could be used Figure 2. The TLC shows this reaction generated many by-products along with the desired target compound (blue spot).

Figure 2. TLC of reaction mixture in 7:3 hexane/ethyl acetate. The product is the large blue spot.
Based on the TLC data, a linear gradient of 7-60% EtOAc over 10 column volumes (CV) was used (10-gram Biotage® Sfär HC column) and generated the results in Figure 3.
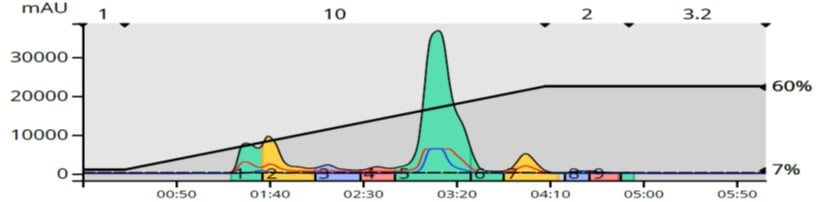 Figure 3. Normal-phase (silica) purification of the reaction mixture shows several separated by-products and one only partially resolved (shoulder on the back of the product peak).
Figure 3. Normal-phase (silica) purification of the reaction mixture shows several separated by-products and one only partially resolved (shoulder on the back of the product peak).
The results using normal-phase silica show a significant shoulder on the back of the product peak. If we take another look at the TLC, you can see another, lighter blue spot just under the main product peak so the flash chromatography result makes sense.
One option to improve this reaction’s purification is to try other normal-phase solvents to change selectivity. While this is a useful approach, changing media from polar (silica) to non-polar (C18), and therefore the solvents from non-polar to polar, usually does a better job at achieving the purification goals.
When purified by reversed-phase (C18) with a 50-100% methanol in water gradient (12-gram Biotage® Sfär C18 column), the results look very different with the product peak well resolved from the major and minor by-products, including the shoulder from the silica column purification, Figure 4.
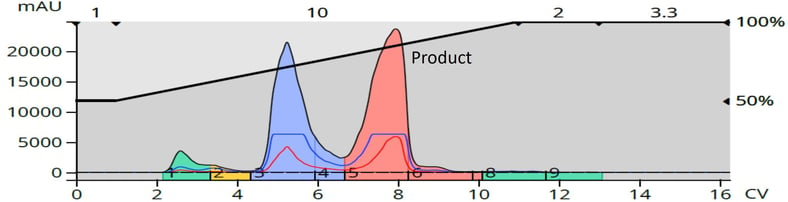 Figure 4. Reversed-phase (C18) purification of reaction mixture product provided superior purification results.
Figure 4. Reversed-phase (C18) purification of reaction mixture product provided superior purification results.
Why did the C18 column provide better chromatography? Well, it comes down to how the reaction mixture's compounds interact with both the mobile phase solvents and the column’s media. In silica column chromatography, compounds tend to elute based on polarity differences with those more polar eluting later. For this reaction mixture, the major by-product had very similar polarity to the reaction product making its separation difficult.
With reversed-phase, the interactions are based more on hydrophobicity differences (e.g. number of carbons, etc.). So, with this reaction, the more polar by-product was significantly less hydrophobic than the product and eluted much earlier making the purification a success.
This is just one of many examples where reversed-phase flash chromatography was the better choice for reaction mixture purification. It is also a greener purification strategy since the solvents are water-compatible and the columns are reusable.
Interested in learning more about reversed-phase (C18) flash chromatography? Check out our white-paper How to Determine Reversed-phase Loading Capacity.
Published: Apr 17, 2023 10:08:25 AM

