Automated extraction of opiates from hydrolyzed and non-hydrolyzed urine using ISOLUTE® SLE+
By Biotage
For research use only. NOT for use in diagnostic procedures.
 Figure 1. Structure of 6-monoacetylmorphine.
Figure 1. Structure of 6-monoacetylmorphine.
Introduction
This application note describes the fully-automated extraction of opiates from acid-hydrolyzed urine and non-hydrolyzed urine prior to GC/MS analysis. The methods were automated using Biotage® Extrahera™ configured for use with ISOLUTE® SLE+ supported liquid extraction cartridges.
The simple sample preparation procedure delivers clean extracts and analyte recoveries of greater than 74% with RSDs lower than 10%. Using the Biotage® Extrahera™, 24
hydrolyzed samples were extracted in under 31 minutes and 24 non-hydrolyzed samples were extracted in under 36 minutes. The limits of quantitation, described in Table 2, all meet or exceed the sensitivity requirements set by SAMHSA and EWDTS for workplace testing applications.
ISOLUTE® SLE supported liquid extraction plates and cartridges offer an efficient alternative to traditional liquid-liquid extrac- tion (LLE) for bioanalytical sample preparation, providing high analyte recoveries, no emulsion formation, and significantly reduced sample preparation.
Note: The procedures described in this application note can also be performed manually using a Biotage® PRESSURE+ 48 positive pressure manifold.
Analytes
Dihydrocodeine, codeine, hydrocodone, hydromorphone, morphine, 6-MAM, oxycodone, and oxymorphone.
Internal standards
Morphine-D6, 6-MAM-D6, oxycodone-D3, oxymorphone-D3
Sample preparation procedure
Format
ISOLUTE® SLE+ 1 mL sample capacity cartridges (Tabless), part number: 820-0140-CG
Extraction of hydrolyzed urine samples (if hydrolysis is required)
Sample pre-treatment prior to extraction
Urine hydrolysis
To 5 mL of urine add 50 μL of internal standard solution of concentration 10 ng/μL. Allow equilibration to take place at room temperature for 1 hour. Perform hydrolysis as follows: add concentrated hydrochloric acid (3 drops). Cap the vial and heat in a water bath for 20 minutes at 60 degrees. Allow to cool and add 3 drops of concentrated ammonium hydroxide to achieve approximately pH 9.
Transfer 1 mL to a 75 x 12 mm tube for automated extraction using Biotage® Extrahera™.
Extraction procedure performed by Biotage® ExtraheraTM
Sample loading
Load 0.5 mL of the sample containing internal standard onto the SLE cartridge and apply a pulse of positive pressure is applied to initiate flow. Allow the sample to absorb for 5 minutes.
Analyte extraction (Elution)
Apply DCM:IPA (95:5, v:v, 4 mL) to the cartridge and allow to flow under gravity for 5 minutes into 60 x 18 mm rimless glass tubes. Apply positive pressure to push through any remaining extraction solvent prior to evaporation.
Extraction of non-hydrolyzed urine samples (if hydrolysis is not required)
Sample pre-treatment prior to extraction
To 1 mL of urine add 10 μL of internal standard solution of concentration 10 ng/μL. Allow equilibration to take place at room temperature for 1 hour. Transfer this volume to a 75 x 12 mm tube for Extrahera™ automation.
Extraction procedure performed by Biotage® ExtraheraTM
Sample loading
Add 1 mL of ammonium hydroxide (0.1% aq) to the urine sample containing internal standard.
Load 1 mL of this pre-treated sample onto the SLE cartridge and apply a pulse of positive pressure to initiate flow. Allow the sample to absorb for 5 minutes.
Analyte extraction (Elution)
Apply 4 mL of DCM:IPA, 95:5 (v:v) to the cartridge and allow to flow under gravity for 5 minutes into 60 x 18 mm rimless glass tubes. Apply positive pressure to push through any remaining extraction solvent prior to evaporation.
Post elution and derivatization
Note: The same procedure is used for both hydrolyzed and non-hydrolyzed urine samples.
Evaporate the extracts using a TurboVap® set to 1.2 Liters/ minute flow for 20 mins, with the water temperature set to 40 °C. Upon dryness, reconstitute the extracts with 400 μL ethyl acetate and vortex for 10 seconds before transferring to high recovery GC vials.
Evaporate the vials using a TurboVap set to 1.2 Liters/minute flow for 15 mins, with the water temperature set to 40 °C. Alternatively evaporate with a Biotage® SPE Dry set to total flow of 40 Liters/minute with temperature set to 40 °C.
Reconstitute the extracts with 25 µL ethyl acetate and 25 µL BSTFA with 1% TMCS. Alternatively MSTFA with 1% TMCS can be used.
GC conditions
Instrument
Agilent 7890A with QuickSwap
Column
Restek RXi-5ms, 30 m x 0.25 mm ID x 0.25 μm)
Carrier
Helium 1.2 mL/min (constant flow)
Inlet
280 °C, Splitless, purge flow: 50 mL/min at 1.0 min
Injection
1 µL
Wash solvents
Methanol and ethyl acetate
Oven
Initial temperature 80 °C, hold for 1 minute Ramp 16 °C/min to 265 °C, hold for 4.5 minutes Ramp 50 °C to 330 °C
Post run
Backflush for 1.6 minutes (2 void volumes)
Transfer line
280 °C
Mass spectrometry conditions
Instrument
Agilent 5975C
Source
230 °C
Quadrupole
150 °C
MSD mode
SIM
SIM parameters
Table 1. Ions acquired in the Selected Ion Monitoring (SIM) mode.
|
SIM Group |
Analyte |
Target (Quant) Ion |
Qualifier Ion |
|
1 |
Dihydrocodeine |
373 |
146 |
|
2 |
Codeine |
371 |
196 |
|
2 |
Hydrocodone |
299 |
242 |
|
3 |
Morphine-D6 |
435 |
293 |
|
3 |
Morphine |
429 |
287 |
|
3 |
Hydromorphone |
357 |
300 |
|
4 |
6-MAM-D6 |
405 |
210 |
|
4 |
6-MAM |
399 |
204 |
|
4 |
Oxycodone-D3 |
390 |
375 |
|
4 |
Oxycodone |
387 |
372 |
|
4 |
Oxymorphone-D3 |
448 |
433 |
|
4 |
Oxymorphone |
445 |
430 |
Results
Analyte recovery, reproducibility, linearity and cleanliness studies were performed using intact urine from healthy volunteers.
Recovery data is shown in Figure 2.This demonstrates that the protocol provides extraction recovery of 86%-101% from acid-hydrolyzed urine and 75%–93% from non-hydrolyzed urine. RSD values were less than 10% across both methods. Figure 2. Typical analyte % extraction recoveries for hydrolyzed urine (top) and non-hydrolyzed urine (bottom) respectively.
Figure 2. Typical analyte % extraction recoveries for hydrolyzed urine (top) and non-hydrolyzed urine (bottom) respectively.
Following recovery determination, calibration curves were constructed with urine spiked before hydrolysis at concentrations 5, 10, 50, 100, 500, 1000 and 2000 ng/mL. Representative curves are shown in Figure 3.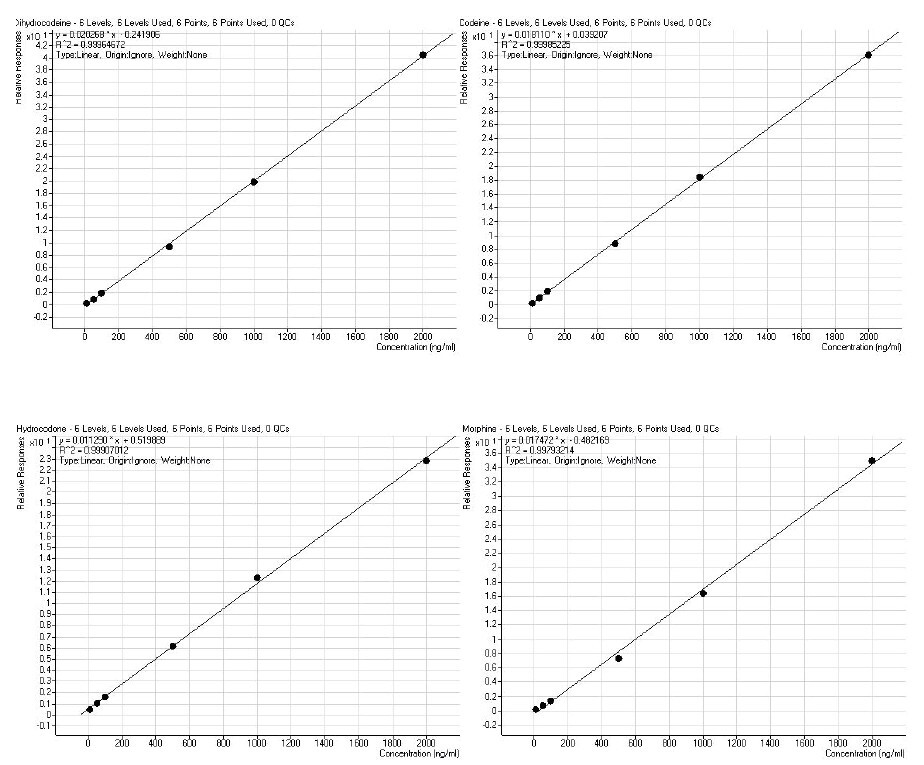
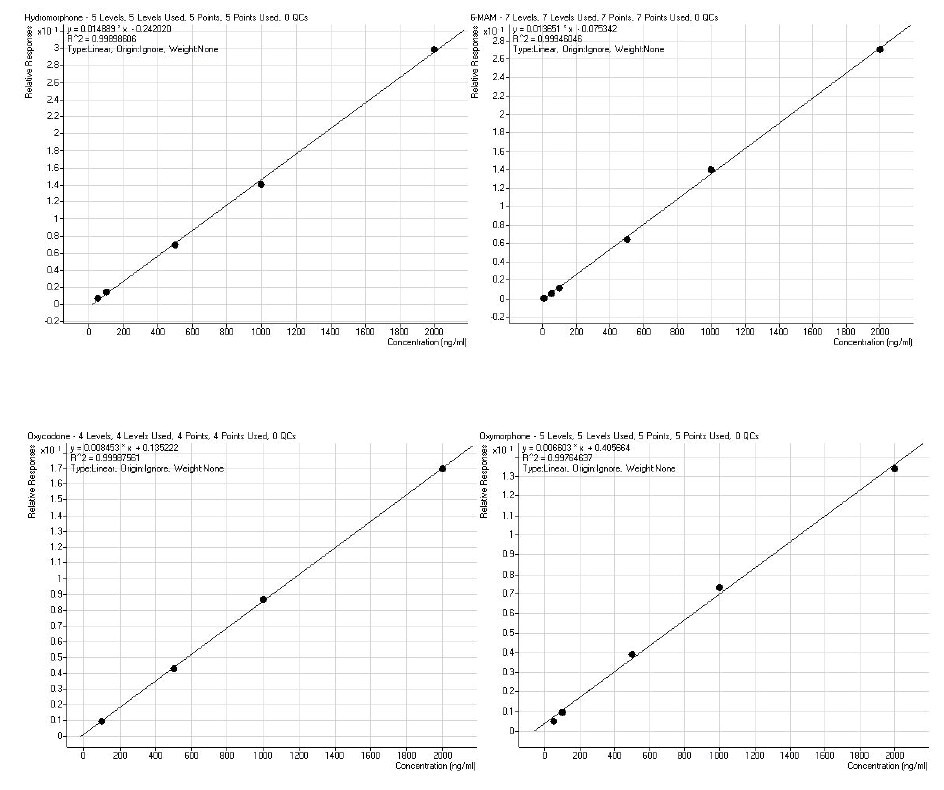
Figure 3. Calibration curves of the application analytes constructed following automated extraction of acid-hydrolyzed urine using ISOLUTE® SLE+ with the optimal protocol. Analyte concentrations visible here are 5, 10, 50, 100, 500 1000 and 2000 ng/mL showing r2 values of greater than 0.99. Internal standard concentrations are at 100 ng/mL.
The analyte signal allows an approximate inferred limit of quantitation of between 5 and 60 ng/mL, satisfying the typical workplace drug testing requirements.
Table 2. Limits of quantitation for the application analytes.
|
Analyte |
Non-hydrolyzed limit of quantitation, inferred from lowest calibrator (ng/mL) |
Acid-hydrolyzed limit of quantitation, inferred from lowest calibrator (ng/mL) |
|
Dihydrocodeine |
6 |
10 |
|
Codeine |
7.5 |
10 |
|
Hydrocodone |
10 |
10 |
|
Hydromorphone |
10 |
10 |
|
Morphine |
20 |
22 |
|
6-MAM |
5 |
5 |
|
Oxycodone |
60 |
55 |
|
Oxymorphone |
28 |
25 |
Chemicals and reagents
- Reference standards (including deuterated internal standards), ammonium acetate (reagent grade ≥98%), concentrated ammonium hydroxide (~15M), concentrated hydrochloric acid (~12M) and derivatization agents were purchased from Sigma- Aldrich Company Ltd. (Gillingham, UK).
- HPLC-grade solvents (acetonitrile, methanol, DCM) were purchased from Honeywell Research Chemicals (Bucharest, Romania).
- Water used was 18.2 MOhm-cm, drawn daily from a Direct-Q5 water purifier.
- 0.1% ammonium hydroxide (aq) is prepared with 50 µL of commercially available 28–30% ammonia hydroxide and 49.95 mL of deionized water.
- Concentrated hydrochloric acid used to perform hydrolysis is commercially available 12M concentration.
- An alternative derivatization approach is the use of MSTFA with 1% TMCS.
Additional information
All data shown in this application note was generated using real, intact matrix, obtained from human volunteers.
Ordering information
|
Part Number |
Description |
Quantity |
|
820-0140-CG |
ISOLUTE® SLE+ 1 mL Sample Volume (Tabless) |
30 |
|
414001 |
Biotage® Extrahera™ |
1 |
|
PPM-48 |
Biotage® PRESSURE+ 48 Positive Pressure Manifold |
1 |
|
415000 |
TurboVap® LV |
1 |
|
SD-9600-DHS-EU |
Biotage® SPE Dry 96 Sample Evaporator 220/240 V |
1 |
|
SD-9600-DHS-NA |
Biotage® SPE Dry 96 Sample Evaporator 100/120 V |
1 |
Literature Number: AN918

