Automated extraction of per- and polyfluoroalkyl substances (PFAS) from human urine using Biotage® Extrahera™ LV-200 and ISOLUTE® PLD+ for PFAS Plates Prior to LC-MS/MS
By Biotage
For research use only. NOT for use in diagnostic procedures.
Per and polyfluoroalkyl substances (PFAS) comprise many compounds that occur in a broad range of matrices and environments. PFAS are of concern because of their high persistence, bioaccumulation and slow elimination, and impacts on human and environmental health. Exposure to PFAS correlates with changes in metabolism, higher cholesterol, and increased risk of some cancers. PFAS pose challenges in the analytical laboratory as they are present in common consumables and hardware. The method described in this application note can be used to reproducibly quantitate multiple PFAS classes with low matrix effects at clinically relevant levels in human urine. Figure 1. Selected PFAS structures
Figure 1. Selected PFAS structures
Introduction
This application note describes the extraction of thirty-one PFAS from human urine using Biotage® ISOLUTE® PLD+ for PFAS plates prior to UHPLC-MS/MS analysis.
The sample preparation procedure simultaneously removes unwanted matrix components from urine, maintaining high, reproducible analyte recoveries, and minimizing matrix interferences. ISOLUTE® PLD+ for PFAS can be integrated quickly and easily into routine workflows, increasing productivity and reducing instrument downtime. No evaporation step is needed, extracts are diluted with compatible solvent prior to injection.
The application note includes optimized conditions for automated processing of ISOLUTE® PLD+ for PFAS plates (using the Biotage® Extrahera™ LV-200, see appendix for settings) and manual processing (using the Biotage® VacMaster™-96 vacuum manifold). Data generated using both processing systems is shown.
Analytes
A wide range of PFAS classes were selected to reflect the broad applicability of this simple sample preparation approach.
Table 1. Target PFAS Analytes
|
Analyte |
Abbreviation |
CAS No. |
|
Perfluoropentanoic acid |
PFPeA |
2706-90-3 |
|
Perfluorohexanoic acid |
PFHxA |
307-24-4 |
|
Perfluoroheptanoic acid |
PFHpA |
375-85-9 |
|
Perfluorooctanoic acid |
PFOA |
335-67-1 |
|
Perfluorononanoic acid |
PFNA |
375-95-1 |
|
Perfluorodecanoic acid |
PFDA |
335-76-2 |
|
Perfluoroundecanoic acid |
PFUdA |
2058-94-8 |
|
Perfluorododecanoic acid |
PFDoA |
307-55-1 |
|
2H-Perfluoro-2-decenoic acid |
8:2 FTUCA |
70887-84-2 |
|
Perfluorobutanesulfonic acid |
PFBS |
375-73-5 |
|
Perfluoropentanesulfonic acid |
PFPeS |
2706-91-4 |
|
Perfluorohexanesulfonic acid |
PFHxS |
355-46-4 |
|
Perfluoroheptanesulfonic acid |
PFHpS |
375-92-8 |
|
Perfluorooctanesulfonic acid |
PFOS |
1763-23-1 |
|
Perfluorodecanesulfonic acid |
PFDS |
335-77-3 |
|
4:2-Fluorotelomer sulfonic acid |
4:2FTS |
757124-72-4 |
|
6:2-Fluorotelomer sulfonic acid |
6:2FTS |
27619-97-2 |
|
8:2-Fluorotelomer sulfonic acid |
8:2FTS |
39108-34-4 |
|
Perfluoro-3-oxapentane-sulfonic acid |
PFEESA |
113507-82-7 |
|
Perfluoro-3-methoxypropanoic acid |
PFMPA |
377-73-1 |
|
Perfluoro-4-methoxybutanoic acid |
PFMBA |
863090-89-5 |
|
Perfluoro-2-propoxypropanoic acid |
GenX |
13252-13-6 |
|
Perfluoro-3,6-dioxaheptanoic acid |
NFDHA |
151772-58-6 |
|
Dodecafluoro-3H-4,8-dioxanonanoic acid |
ADONA |
919005-14-4 |
|
Perfluorooctanesulfonamide |
PFOSA |
754-91-6 |
|
N-Methyl perfluorooctanesulfonamido acetic acid |
Me-PFOSAA |
2355-31-9 |
|
N-Ethyl perfluorooctanesulfonamido acetic acid |
Et-PFOSAA |
2991-50-6 |
|
N-Methylperfluorooctanesulfonamide |
N-MeFOSA |
31506-32-8 |
|
N-Ethylperfluorooctanesulfonamide |
N-EtFOSA |
4151-50-2 |
|
9-chlorohexadecafluoro-3-oxanonanesulfonic acid (F-53B) |
6:2 Cl-PFESA |
756426-58-1 |
|
11-chloroeicosafluoro-3-oxaundecanesulfonic acid (F-53B) |
8:2 Cl-PFESA |
763051-92-9 |
Sample preparation procedure
Format
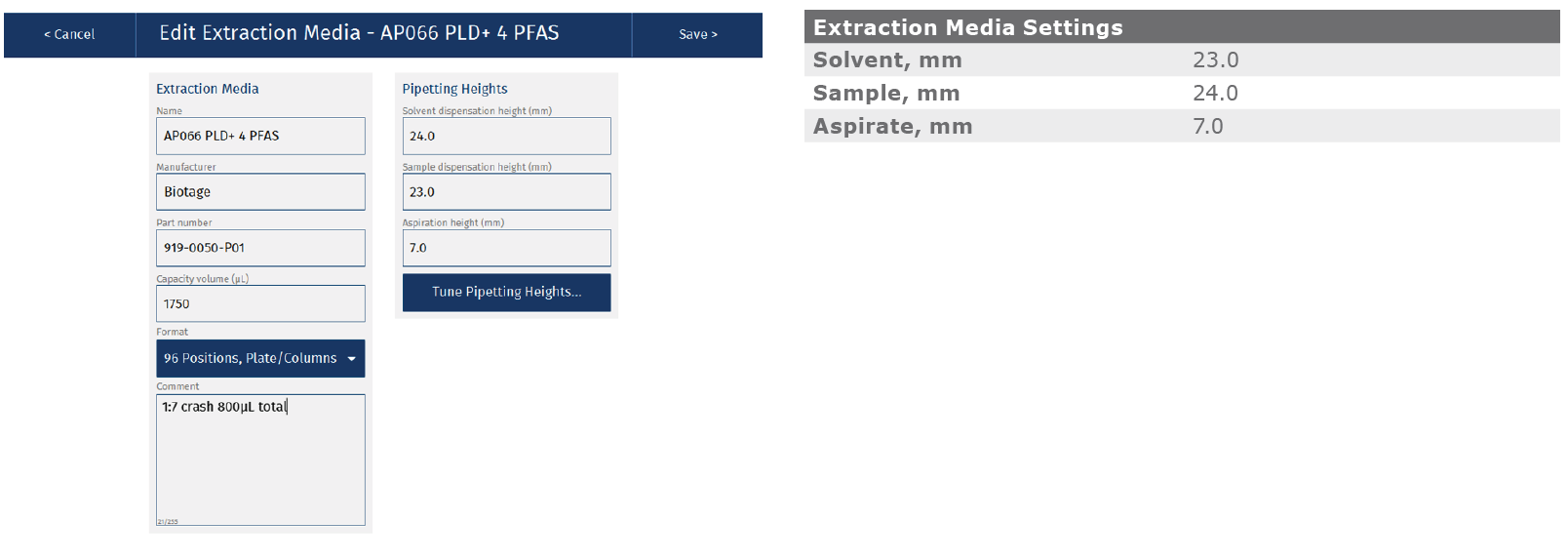
ISOLUTE® PLD+ for PFAS Plate, part number 919-0050-P01
Processing
Samples were processed using a Biotage® Extrahera™ LV-200 automated sample preparation workstation, or manually using a Biotage® VacMaster™ 96 sample processing manifold.
Note: ISOLUTE® PLD+ for PFAS plates can also be processed using the Biotage® Pressure+96 Positive Pressure Manifold. Processing parameters are available on request.
Sample pre-treatment
Approximately 150 µL of urine (sample) from anonymized healthy human volunteers was added to each well of a 2 mL square collection plate for automated or manual processing.
If used, add internal standard at this stage. For example, an appropriate concentration in 10 µL methanol or acetonitrile.
Extraction procedure
Extraction is performed using ISOLUTE® PLD+ for PFAS using a 7:1 solvent to sample ratio with ‘solvent first’ methodology.
Biotage® Extrahera™ LV-200:
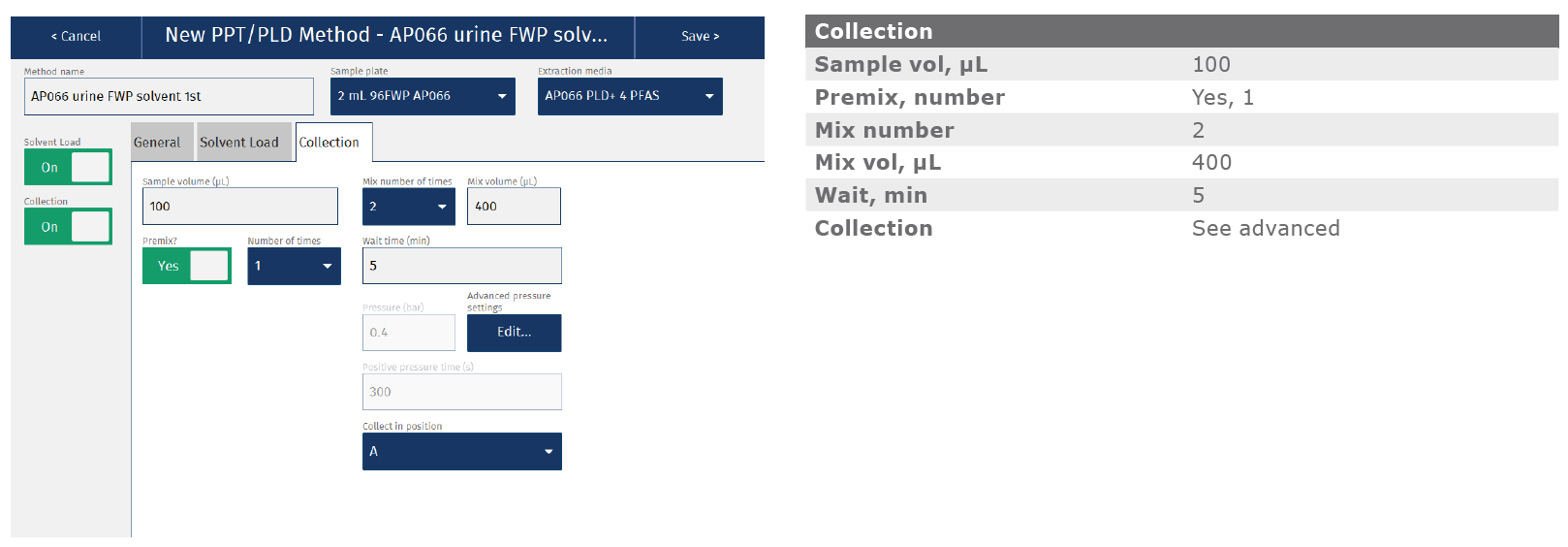
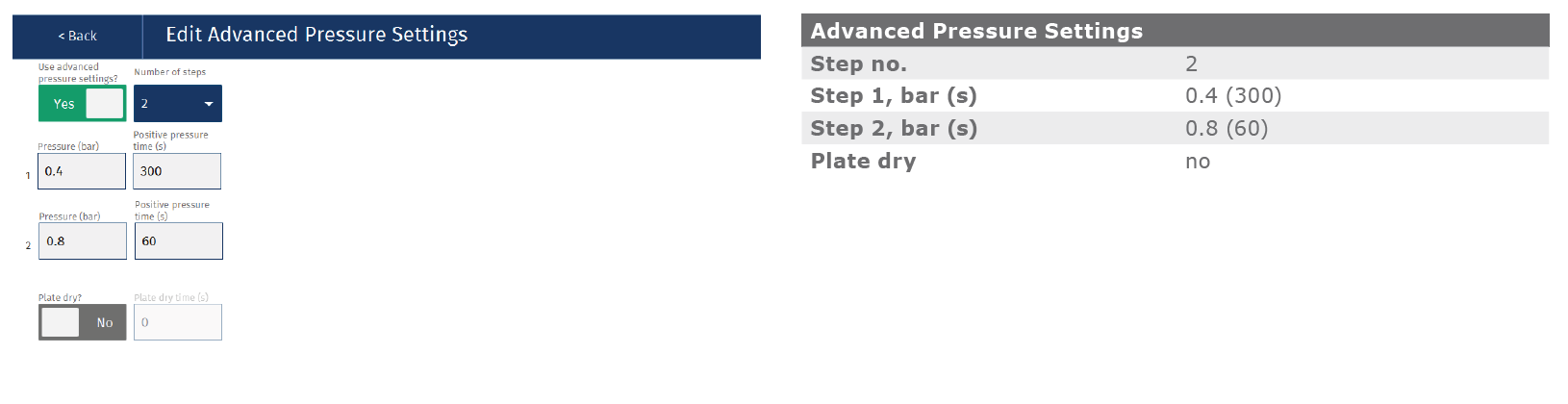
Dispense 700 μL of acetonitrile (MeCN) extraction solvent in each well. Dispense 100 µL of urine sample into each well. Mix the solvent and sample with 2x aspirate/dispense cycles and wait 5 minutes. Process the plate by applying 0.4 Bar (5 min), 0.8 Bar (1 min). Collect the extracts in a 2 mL square collection plate. See Appendix 1 for additional details.
Biotage® VacMaster™-96:
Using a multi-channel pipette (or similar), dispense 700 μL of acetonitrile (MeCN) extraction solvent in each well. Dispense 100 µL of urine sample vertically into each well with force. Mix the solvent and sample with 5x aspirate/dispense cycles and wait 5 minutes. Process the plate by applying -0.2 Bar (5 min), -0.4 Bar (to complete). Collect the extracts in a 2 mL square collection plate.
Note: This process can be scaled down to for a reduced urine sample volume of 50 µL. 350 µL of acetonitrile should be used as extraction solvent, following the same procedure. A summary of method performance for 50 µL samples can be found in table 4 (automated method) and table 5 (manual method).
Post extraction
Using a multi-channel pipette (or similar), dispense 200 µL of extracted sample to each well of a new 2 mL square collection plate. Dilute the contents of each well with 200 μL of 20 mM ammonium acetate (aq) dilution solvent. Vortex the plate gently for 30 s to mix the contents and cover with a pierceable sealing cap before transferring to the LC-MS/MS system for analysis.
Analytical conditions
U/HPLC parameters
-
Instrument: Shimadzu Nexera UHPLC using fluoropolymer-free tubing and a pre-injector trap column (Restek PFAS Delay 50 x 2.1 mm).
-
Column: Avantor ACE Ultracore SuperC18 2.5 µm (50 x 2.1 mm) with a Raptor ARC-18 EXP guard 2.7 µm 5 x 2.1 mm
-
Mobile phase A: 5 mM ammonium acetate (aq)
-
Mobile phase B: 5 mM ammonium acetate in MeOH
-
Flow rate: 0.4 mL/min
-
Column temperature: 40 °C
-
Injection volume: 5 µL (no rinsing)
-
Sample temperature: 15 °C
MS/MS parameters
- Instrument: AB Sciex 5500 triple quadrupole system operating in negative ion mode
- Ionspray voltage: -3500 V
- Source temperature: 500 °C
- Curtain gas: 40 psi
- Source gases: GS1 40 psi / GS2 60 psi
Table 2. Gradient parameters
|
Time / min |
% B |
Divert |
|
0.1 |
30 |
|
|
0.5 |
|
MS |
|
6.0 |
95 |
|
|
7.5 |
|
waste |
|
7.6 |
95 |
|
|
7.7 |
30 |
|
|
9.0 |
30 |
|
Table 3. MRM parameters
|
Analyte |
Transition, m/z |
DP |
CE |
|
PFPeA |
263 > 218.9 |
-50 |
-12 |
|
PFHxA |
313 > 268.9 |
-50 |
-13 |
|
PFHpA |
363.1 > 318.8 |
-50 |
-12 |
|
PFOA |
413.1 > 368.8 |
-50 |
-14 |
|
PFNA |
463 > 418.8 |
-50 |
-15 |
|
PFDA |
513 > 468.8 |
-50 |
-16 |
|
PFUdA |
563 > 518.8 |
-50 |
-16 |
|
PFDoA |
613 > 568.85 |
-50 |
-18 |
|
8:2 FTUCA |
457.1 > 392.9 |
-50 |
-21 |
|
PFBS |
298.9 > 80 |
-100 |
-64 |
|
PFPeS |
348.9 > 79.95 |
-100 |
-72 |
|
PFHxS |
399 > 79.95 |
-100 |
-86 |
|
PFHpS |
448.9 > 79.95 |
-100 |
-94 |
|
PFOS |
498.9 > 79.95 |
-100 |
-110 |
|
PFDS |
598.9 > 79.95 |
-100 |
-123 |
|
4:2FTS |
327 > 80.95 |
-100 |
-56 |
|
6:2FTS |
427 > 81 |
-100 |
-72 |
|
8:2FTS |
526.9 > 80.95 |
-100 |
-82 |
|
PFEESA |
314.9 > 134.9 |
-50 |
-32 |
|
PFMPA |
229 > 85 |
-50 |
-18 |
|
PFMBA |
278.9 > 85 |
-50 |
-14 |
|
GenX |
328.9 > 168.8 |
-50 |
-16 |
|
NFDHA |
294.9 > 200.9 |
-100 |
-14 |
|
ADONA |
377.1 > 250.9 |
-50 |
-28 |
|
PFOSA |
497.9 > 77.9 |
-100 |
-30 |
|
Me-PFOSAA |
569.8 > 418.8 |
-100 |
-28 |
|
Et-PFOSAA |
583.9 > 418.9 |
-100 |
-30 |
|
N-MeFOSA |
511.9 > 218.9 |
-100 |
-34 |
|
N-EtFOSA |
525.9 > 218.9 |
-100 |
-36 |
|
6:2 Cl-PFESA |
530.9 > 350.8 |
-100 |
-38 |
|
8:2 Cl-PFESA |
630.9 > 450.7 |
-100 |
-43 |
Results
This application note was developed using pooled human urine. Further optimisation may be required for other similar matrices (for example, to compensate for regional dietary variation). Recovery data shown in this application note was generated using intact, non-stripped, representative matrix.
Extraction recovery was determined using a 160 pg (1.6 ng/ mL extracted) spike before extraction as a proportion of the spike after extraction (fort). The spike area response was used to determine extraction repeatability as % RSD (n=6). Matrix effects were estimated for each analyte using the fort as a proportion of a dilute standard at the same concentration (0.5 pg on-column). Blank contributions were estimated for each analyte using the blank response (n=3) as a proportion of the dilute standard (0.5 pg on-column).
Recovery and reproducibility: automated method
Typical analyte recovery processed using Extrahera™ LV-200 was between 71% and 82% for a 100 µL sample load. Extraction repeatability using the system is typically less than 5% RSD (figure 2).
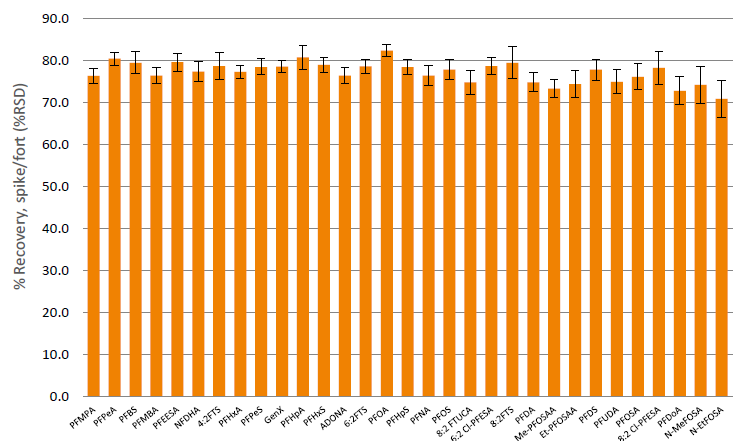
Figure 2. Recovery (%RSD (n=6) shown as error bars) of PFAS analytes from 100 µL urine (spiked at a concentration of 1.6 ng/mL), using the method described in this application note.
Typical matrix factors for samples processed using Extrahera™ LV-200 were between 1.2 and 1.5 (figure 3), slightly higher than blood products e.g. serum. Matrix effect factors for PFPeA, and PFOS are elevated above 1.5 due to contribution of these PFAS species present in the blank (non-stripped) urine.
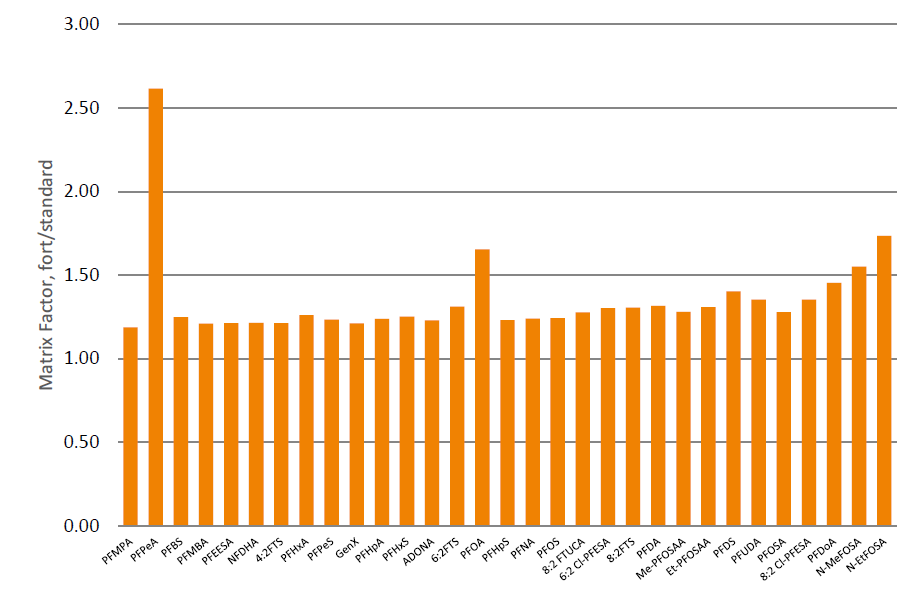
Figure 3. Typical matrix factors for 100 µL urine processed using Biotage® Extrahera™ LV-200 relative to 1.6 ng/mL. This includes contribution from the sample matrix (pooled human urine, non-stripped), plus any from processing using Extrahera™ LV-200, ISOLUTE® PLD+ for PFAS plate and reagents.
Matrix blank factors for PFOA, PFHxS, and PFOS were between 0.01 and 0.15 (shown in figure 4). This matrix-derived contribution corresponds to between 0.02 and 0.24 ng/mL extracted sample (analyte dependent).
However, processing residues using ISOLUTE® PLD+ for PFAS are extremely low, as demonstrated by figure 5. This shows the combined contribution of PFAS species derived from the ISOLUTE® PLD+ for PFAS, processing using Extrahera™ LV-200, and the reagents used as described in this application note (with no matrix present), estimated as equivalent to ~22 pg/mL sample.
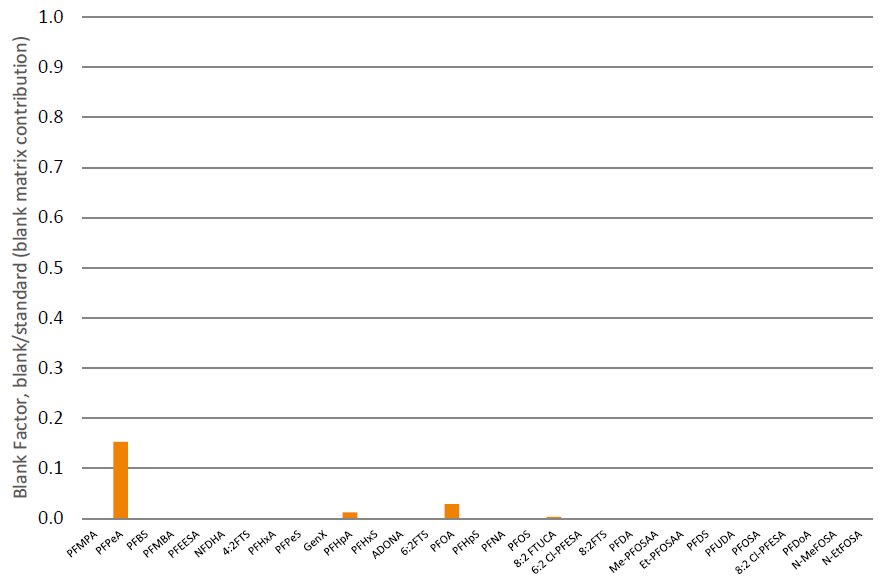
Figure 4. Matrix-derived blank contribution corresponds to between 0.02 and 0.24 ng/mL extracted sample (figure 4). Note: matrix consists of pooled human urine, suggesting that PFAS residues of up to 0.24 ng/mL (analyte dependent) are present in the sample matrix.
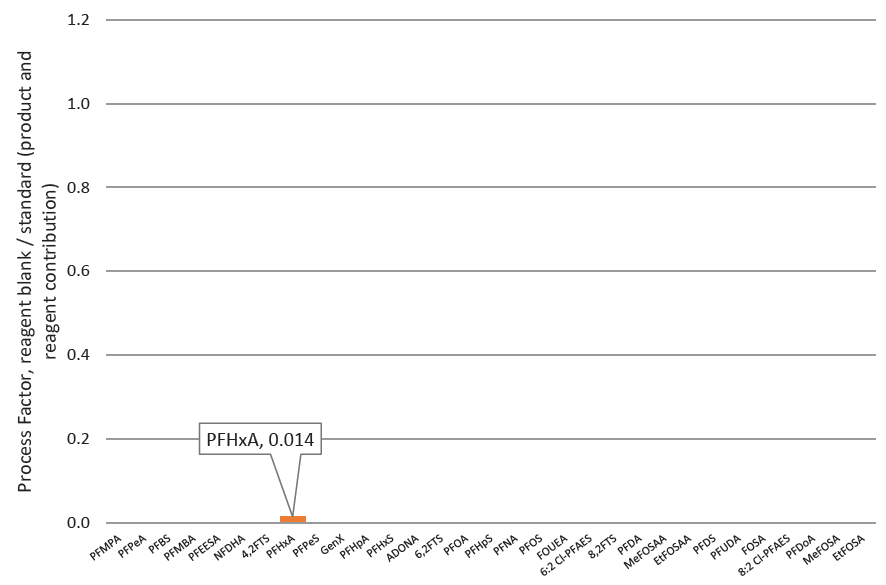
Figure 5. Contribution to the blank of PFAS species related to the ISOLUTE® PLD+ for PFAS extraction product, automated processing and reagents– with no matrix present. A process factor of 0.014 was determined, equivalent to ~22 pg/mL.
Recovery and reproducibility: manual method
Typical analyte recovery processing manually using vacuum was between 77% and 86% for a 100 µL sample load (data not shown). Manually processed extraction repeatability
was typically less than 8% RSD. Matrix factors were typically between 1.2 and 1.5. Blank factors were less than 0.01, equiva- lent to between 0.02 ng/mL extracted sample. Process factors are similar to automated methods.
Automated vs Manual processing
PFAS recovery and matrix factors when extracted using ISOLUTE® PLD+ for PFAS are comparable between automated processing using the Extrahera™ LV-200 and manual processing using a VacMaster™-96. Automated processing using the Extrahera demonstrates improved repeatability compared to manual processing.
Linearity and LOQ
Method performance was evaluated using external standards extracted from spiked matrix over 8 levels from 0.1 ng/mL to 100 ng/mL (n=4). Limit of quantitation (LOQ) was estimated as the lowest extracted concentration with signal/noise (S/N) > 10-20. Analyte linearity was determined acceptable where: the calibration coefficient (r2) was > 0.995; S/N > 10-20 (estimated using Analyst 1.6.3, peak-to-peak); repeatability < 10% RSD (< 15% at LOQ), with accuracy 90-110% (80-120% at LOQ).
A representative chromatogram of matrix extracted standards spiked at 2 ng/mL is shown in figure 5. All extracted analytes demonstrate good separation and peak shape.
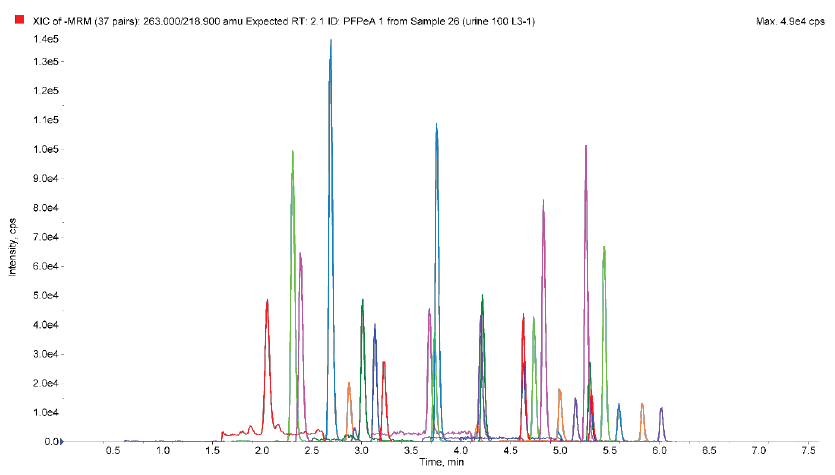
Figure 6. Representative PFAS chromatogram, extracted urine, 2 ng/mL spike
Representative calibration curves are shown below (figures 7 & 8, automated and manual processing respectively). Method performance for all analytes is tabulated below (table 4 & 5, automated and manual processing respectively). Most analytes demonstrate LOQ at 0.1 ng/mL. However, some perfluorocabox- ylic acid and perfluorosulfonic acid LOQ are 0.4 ng/mL. All analytes demonstrate good linearity, r2 > 0.995. The majority of analytes demonstrate repeatability < 10% at all calibration levels (< 15% at LOQ). Typical analyte accuracy was 90-110% (80-120% at LOQ).
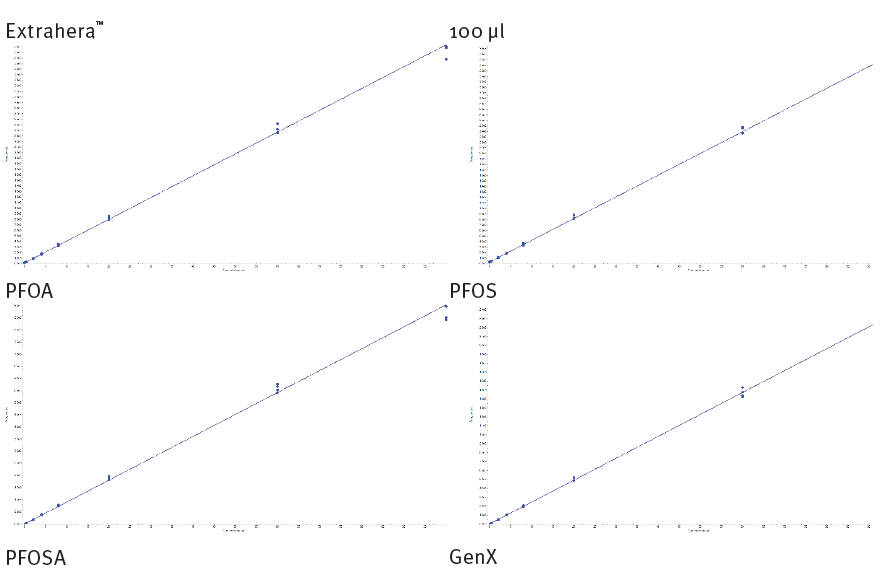
Figure 7. Calibration curves for a) PFOA b) PFOS c) PFOSA and d) GenX extracted from 100 µL of urine using Biotage® Extrahera™ LV-200
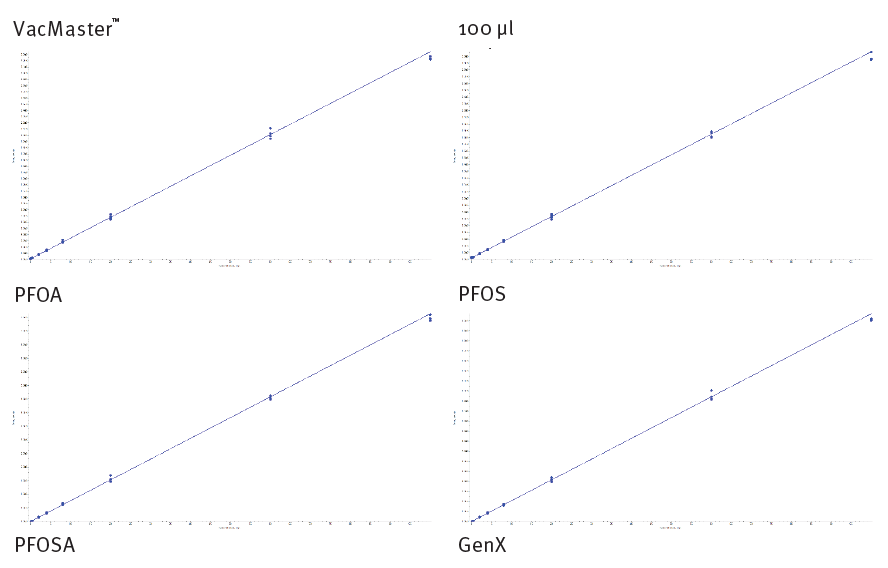
Figure 8. Calibration curves for a) PFOA b) PFOS c) PFOSA and d) GenX extracted from 100 µL of urine using Biotage® VacMaster™ -96
Table 4. Method performance for the automated method (100 µL and 50 µL sample volumes)
|
Extrahera |
|
100 µL |
|
|
|
|
50 µL |
|
|
|
|
Analyte |
r² |
LOQ, ng/mL |
S/N |
RSD % |
Accuracy % |
r² |
LOQ, ng/mL |
S/N |
RSD % |
Accuracy % |
|
PFPeA |
0.9986 |
0.4 |
29 |
7.7 |
97-107 |
0.9980 |
0.1 |
13 |
8.7 |
97-109 |
|
PFHxA |
0.9982 |
0.4 |
13 |
6.9 |
98-106 |
0.9978 |
0.1 |
21 |
7.0 |
97-112 |
|
PFHpA |
0.9982 |
0.4 |
17 |
7.1 |
97-105 |
0.9986 |
0.4 |
18 |
5.9 |
97-103 |
|
PFOA |
0.9978 |
0.4 |
36 |
8.1 |
99-109 |
0.9982 |
0.4 |
34 |
6.9 |
96-106 |
|
PFNA |
0.9978 |
0.4 |
20 |
8.2 |
94-107 |
0.9980 |
0.4 |
22 |
6.4 |
96-104 |
|
PFDA |
0.9982 |
0.4 |
24 |
8.2 |
98-105 |
0.9980 |
0.4 |
33 |
5.7 |
96-103 |
|
PFUDA |
0.9978 |
0.4 |
27 |
6.9 |
98-107 |
0.9982 |
0.4 |
28 |
5.6 |
97-103 |
|
PFDoA |
0.9980 |
0.4 |
28 |
7.6 |
95-108 |
0.9980 |
0.4 |
27 |
5.6 |
96-103 |
|
8:2 FTUCA |
0.9978 |
0.1 |
70 |
8.9 |
94-105 |
0.9980 |
0.1 |
71 |
5.4 |
96-103 |
|
PFBS |
0.9980 |
0.1 |
75 |
5.9 |
98-109 |
0.9980 |
0.1 |
54 |
7.5 |
98-108 |
|
PFPeS |
0.9978 |
0.1 |
49 |
6.1 |
97-106 |
0.9972 |
0.1 |
27 |
7.0 |
97-105 |
|
PFHxS |
0.9982 |
0.1 |
60 |
8.7 |
97-107 |
0.9978 |
0.1 |
42 |
7.0 |
97-103 |
|
PFHpS |
0.9982 |
0.1 |
99 |
5.8 |
98-106 |
0.9976 |
0.1 |
156 |
9.7 |
98-104 |
|
PFOS |
0.9976 |
0.1 |
89 |
10.5 |
94-105 |
0.9982 |
0.1 |
95 |
5.5 |
96-106 |
|
PFDS |
0.9984 |
0.1 |
59 |
7.0 |
97-105 |
0.9982 |
0.1 |
137 |
8.2 |
98-103 |
|
4:2FTS |
0.9986 |
0.4 |
36 |
7.8 |
98-107 |
0.9978 |
0.4 |
22 |
7.4 |
97-104 |
|
6:2FTS |
0.9978 |
0.1 |
16 |
9.3 |
98-105 |
0.9990 |
0.1 |
17 |
12.2 |
91-101 |
|
8:2FTS |
0.9976 |
0.4 |
41 |
7.4 |
98-106 |
0.9982 |
0.4 |
16 |
8.6 |
91-103 |
|
PFEESA |
0.9980 |
0.1 |
136 |
5.9 |
98-108 |
0.9978 |
0.1 |
162 |
7.3 |
98-107 |
|
PFMBA |
0.9982 |
0.1 |
172 |
9.1 |
98-108 |
0.9980 |
0.1 |
117 |
7.6 |
98-109 |
|
Gen X |
0.9984 |
0.1 |
35 |
6.7 |
97-110 |
0.9978 |
0.1 |
42 |
6.8 |
96-111 |
|
NFDHA |
0.9984 |
0.1 |
53 |
8.9 |
93-106 |
0.9978 |
0.1 |
54 |
6.5 |
96-103 |
|
ADONA |
0.9980 |
0.1 |
102 |
6.1 |
98-107 |
0.9984 |
0.1 |
162 |
5.2 |
98-107 |
|
FOSA |
0.9976 |
0.1 |
95 |
5.4 |
97-108 |
0.9976 |
0.1 |
80 |
6.1 |
98-109 |
|
Me-PFOSAA |
0.9978 |
0.1 |
46 |
8.0 |
92-103 |
0.9982 |
0.1 |
39 |
7.1 |
96-103 |
|
Et-PFOSAA |
0.9984 |
0.4 |
47 |
9.1 |
95-107 |
0.9974 |
0.4 |
40 |
6.4 |
96-105 |
|
N-MeFOSA |
0.9990 |
0.1 |
36 |
7.9 |
89-104 |
0.9972 |
0.1 |
26 |
10.5 |
92-103 |
|
N-EtFOSA |
0.9980 |
0.1 |
30 |
7.4 |
92-103 |
0.9958 |
0.1 |
24 |
9.0 |
93-103 |
|
6:2 Cl-PFESA |
0.9978 |
0.1 |
43 |
6.5 |
98-108 |
0.9980 |
0.1 |
27 |
6.4 |
98-105 |
|
8:2 Cl-PFESA |
0.9976 |
0.1 |
52 |
6.0 |
98-108 |
0.9978 |
0.1 |
57 |
6.2 |
96-106 |
Table 5. Method performance for the manual method (100 µL and 50 µL sample volumes)
|
VacMaster |
|
100 µL |
|
|
|
|
50 µL |
|
|
|
|
Analyte |
r² |
LOQ, ng/mL |
S/N |
RSD % |
Accuracy % |
r² |
LOQ, ng/mL |
S/N |
RSD % |
Accuracy % |
|
PFPeA |
0.9980 |
0.4 |
10 |
7.3 |
97-105 |
0.9958 |
0.4 |
25 |
8.1 |
94-105 |
|
PFHxA |
0.9978 |
0.4 |
10 |
6.5 |
97-105 |
0.9968 |
0.4 |
10 |
7.5 |
94-102 |
|
PFHpA |
0.9980 |
0.4 |
17 |
6.5 |
97-100 |
0.9958 |
0.4 |
17 |
8.5 |
98-104 |
|
PFOA |
0.9974 |
0.1 |
9 |
10.2 |
98-103 |
0.9972 |
0.4 |
24 |
10 |
98-107 |
|
PFNA |
0.9984 |
0.4 |
20 |
7.4 |
97-102 |
0.9970 |
0.4 |
21 |
7 |
95-102 |
|
PFDA |
0.9982 |
0.4 |
27 |
6.0 |
96-102 |
0.9970 |
0.4 |
19 |
7.1 |
95-105 |
|
PFUDA |
0.9984 |
0.4 |
24 |
5.9 |
97-103 |
0.9962 |
0.4 |
14 |
7.3 |
98-107 |
|
PFDoA |
0.9980 |
0.4 |
23 |
7.1 |
99-109 |
0.9958 |
0.4 |
31 |
8.4 |
98-109 |
|
8:2 FTUCA |
0.9976 |
0.1 |
47 |
6.2 |
94-101 |
0.9966 |
0.1 |
54 |
12.2 |
94-104 |
|
PFBS |
0.9972 |
0.1 |
62 |
6.5 |
98-108 |
0.9956 |
0.1 |
67 |
7 |
96-108 |
|
PFPeS |
0.9980 |
0.1 |
48 |
6.6 |
96-105 |
0.9964 |
0.1 |
43 |
7.2 |
97-105 |
|
PFHxS |
0.9976 |
0.1 |
51 |
6.5 |
95-115 |
0.9964 |
0.1 |
42 |
8.4 |
98-106 |
|
PFHpS |
0.9982 |
0.1 |
130 |
5.9 |
97-104 |
0.9962 |
0.1 |
104 |
7.9 |
96-107 |
|
PFOS |
0.9980 |
0.1 |
114 |
8.6 |
92-102 |
0.9968 |
0.1 |
89 |
7.7 |
97-104 |
|
PFDS |
0.9970 |
0.1 |
94 |
12.3 |
93-106 |
0.9968 |
0.1 |
72 |
7.3 |
96-105 |
|
4:2FTS |
0.9956 |
0.4 |
25 |
8.9 |
98-105 |
0.9966 |
0.4 |
25 |
6.9 |
99-107 |
|
6:2FTS |
0.9986 |
0.1 |
14 |
14.6 |
96-103 |
0.9964 |
0.1 |
17 |
10 |
95-105 |
|
8:2FTS |
0.9988 |
0.1 |
12 |
6.9 |
97-108 |
0.9970 |
0.4 |
28 |
7 |
98-108 |
|
PFEESA |
0.9984 |
0.1 |
111 |
6.6 |
98-107 |
0.9968 |
0.1 |
128 |
6.8 |
98-107 |
|
PFMBA |
0.9986 |
0.1 |
137 |
5.9 |
96-102 |
0.9974 |
0.1 |
161 |
6.3 |
91-103 |
|
Gen X |
0.9982 |
0.1 |
67 |
7.7 |
97-107 |
0.9970 |
0.1 |
57 |
6.8 |
97-104 |
|
NFDHA |
0.9980 |
0.1 |
20 |
7.5 |
95-102 |
0.9964 |
0.1 |
59 |
8.1 |
90-102 |
|
ADONA |
0.9980 |
0.1 |
114 |
6.3 |
98-105 |
0.9968 |
0.1 |
141 |
6.9 |
97-105 |
|
FOSA |
0.9974 |
0.1 |
107 |
10.7 |
98-106 |
0.9966 |
0.1 |
79 |
7.7 |
98-107 |
|
Me-PFOSAA |
0.9974 |
0.1 |
28 |
9.4 |
94-105 |
0.9970 |
0.1 |
35 |
7.8 |
91-105 |
|
Et-PFOSAA |
0.9982 |
0.1 |
13 |
6.3 |
99-107 |
0.9968 |
0.4 |
44 |
11.2 |
97-107 |
|
N-MeFOSA |
0.9976 |
0.1 |
38 |
8.7 |
92-102 |
0.9982 |
0.1 |
32 |
9.4 |
93-101 |
|
N-EtFOSA |
0.9984 |
0.1 |
19 |
9.7 |
94-102 |
0.9980 |
0.1 |
26 |
8.2 |
94-101 |
|
6:2 Cl-PFESA |
0.9982 |
0.1 |
54 |
5.4 |
99-106 |
0.9960 |
0.1 |
60 |
8.3 |
96-107 |
|
8:2 Cl-PFESA |
0.9976 |
0.1 |
47 |
12.0 |
91-109 |
0.9968 |
0.1 |
30 |
7.9 |
98-106 |
Extract cleanliness
ISOLUTE® PLD+ for PFAS extraction is an extremely efficient means of endogenous matrix depletion compared to precipita- tion and dilute/shoot. This is demonstrated by the low matrix effects and LOQs obtained using this technique for sample preparation.
Discussion & Conclusion
Using ISOLUTE® PLD+ for PFAS for sample preparation, this application note demonstrates high PFAS recovery and sensitivity with low matrix factors and good repeatability.
The use of a ‘crash and filter’ flow-through strategy incorporating a multifunctional matrix-scavenging sorbent has several advantages compared to alterative solid phase extraction- based processes.
Urine extracted using ISOLUTE® PLD+ for PFAS results in extremely clean extracts, prolonging LC column lifespan and minimizing instrument down time for cleaning and maintenance.
The simplicity of the method, with fewer extraction steps, and no evaporation requirement has additional benefits. Throughput is increased, with up to 96 samples ready for analysis is ~35 minutes when processed using Extrahera™ LV-200, compared with ~ 80 minutes for a WAX based SPE approach. This, combined with reduced reagent preparation time, has significant productivity benefits.
The range of PFAS species that can be effectively extracted using the ISOLUTE® PLD+ for
PFAS approach is extended. Elimination of the evaporation step means that losses of sulfonamide and substituted sulfonamide PFAS, which can demonstrate low or no recovery following evaporation and reconstitution, are reduced. Poor recoveries of longer chain PFAS associated with WAX based catch and release SPE are also eliminated.
The ubiquitous nature of PFAS meant that the sample matrix (non-stripped pooled human urine) used to generate data in this application note was found to contain up to 1.6 ng/mL of some PFAS species. However, we found that the ISOLUTE® PLD+ for PFAS plate, processing system and reagents typically contrib- uted ~22 pg/mL, making it suitable for determination of PFAS in urine samples at clinically relevant levels.
Chemicals and reagents
- PFAS stock standards were purchased from Wellington Laboratories (Guelph, Canada). The suite contains 10 classes of PFAS, varying by functionality, including: carboxylic acids, sulfonic acids and telomers, sulfonamides, and ethoxy compounds.
- Mixed intermediate standards were prepared from the individual stocks on a class by class basis e.g. carboxylic acids. The intermediate standards were prepared in the appropriate solvent indicated on the stock certificate of analysis (e.g. carboxylic acids require 0.4 mole equivalent of base to prevent formation of methyl esters). Some stocks are sold as potassium or sodium salts e.g. sulfonic acids. A correction should be applied to the dilution to calculate the final concentration of free acid in the mixed intermediate. The intermediate stocks were prepared at 4 µg/mL and stored at -20 °C in 1.5 mL polypropylene vials.
- A mixed working standard was prepared weekly at 160 ng/mL in MeOH from the mixed intermediates and stored at -20 °C in 1.5 mL polypropylene vials. As a guide, a 1/25 dilution can be prepared using: 40 µL x 4 µg/mL / 1000 µL = 160 ng/mL.
- Reagents were purchased from Merck Life Science (Gillingham, UK). LC/MS grade solvents were from Rathburn Chemicals Ltd. (Walkerburn, UK).
- Water (18.2 MΩ.cm) was drawn fresh daily from a Direct-Q 5 water purifier (Merck Life Science UK, Watford, UK).
- All reagents were prepared fresh daily in 250 mL polypropylene bottles.
- Ammonium acetate containing reagents were prepared from a 5 M NH4OAC stock (Sigma-Aldrich ammonium acetate solution, 09691-100ML) stored at 2-8 °C.
- 20 mM NH4OAC was prepared by diluting 1 mL 5 M stock in 250 mL freshly drawn water.
- Mobile phases A and B were prepared by diluting 0.5 mL 5 M NH4OAC in 500 mL water or MeOH respectively.
- Urine samples were obtained from anonymized healthy human volunteers.
Additional information
- Additional precautions were taken to minimize the possibility of system contamination from lab and environmentally derived PFAS. Care should be taken with all consumables, in-house studies have demonstrated consumables manufactured from similar materials can have differing PFAS residue profiles.
- Contact with glass surfaces was minimized as PFAS bind to glass and may be released or transferred during subsequent processing. Consumables used were restricted to virgin polypropylene (PP) or high-density polyethylene (HDPE) where possible. Disposable nitrile gloves were worn at all times and changed frequently.
- Additional triplicate blank analyses were acquired during a typical experiment to determine potential sources of interference: a) solvent blank, freshly prepared solvents in the final 2 mL collection plate in the same proportions as the final dilution (ACN 1:1 20 mM NH4OAC); b) consumable blank, extraction media processed using freshly drawn water as the sample in place of matrix; c) matrix blank, unspiked sample matrix.
- Biotage® Extrahera™ LV-200 solvent inlets were flushed with 4x 20 mL MeOH followed by 4x 20 mL ACN prior to use.
- The UHPLC inlet was modified for the determination of PFAS (see below, figure 9). FEP tubing was replaced with PEEK tubing where possible. PTFE-containing mobile phase
filters were replaced with stainless steel filters. The vacuum degassers were bypassed. A PFAS delay column was installed between the pumps and injector. The inlet was flushed with methanol at 0.4 mL/min and 50 °C for 4 hours prior to use. The injector was flushed with methanol prior to use. Mobile phase and wash solutions are replaced daily.
Optional evaporation/reconstitution conditions
For increased analytical sensitivity the extract can be evaporated and reconstituted in a lower volume of solvent, suitable for injection to the analytical system. The following conditions are suggested:
Following elution, to each well, add 10 μL of keeper solvent (we recommend DMF, but DMSO or ethylene glycol may also be appropriate).
Transfer the collection plate to a TurboVap®-96 Dual evaporation system, and evaporate to constant volume (approximately 10 µL) at 40 oC using air or nitrogen at a flow rate of 36-60 L/min and a plate height 50 mm or greater.
Reconstitute in 10 mM ammonium acetate/methanol (1:1, v/v, 400 µL)
 Figure 9. Modification to the UHPLC inlet, used for PFAS analysis.
Figure 9. Modification to the UHPLC inlet, used for PFAS analysis.
Ordering information
|
Extraction Consumables |
||
|
Part Number |
Description |
Qty |
|
919-0050- P01 |
ISOLUTE® PLD+ for PFAS Plate |
1 |
|
121-5203 |
Collection Plate, 2 mL, square |
50 |
|
Biotage® Extrahera™ LV-200 Processing System, Consumables & Accessories |
||
|
Part Number |
Description |
Qty |
|
417000 |
Biotage® Extrahera™ LV-200 |
1 |
|
414141 |
Biotage Disposable Tips 1000 μL Clear |
1pack (10 x 96 tips) |
|
416444 |
Biotage Disposable Tips 1000 μL Wide Bore, Clear |
1 pack (10 x 96 tips) |
|
414045SP |
Solvent Reservoir 25 mL |
25 |
|
414579 |
Solvent Safety Kit (inc. GL45 Caps, Filters and Bottles*, Qty 5) |
1 |
|
*Polypropylene alternative to 500 mL GL45 bottle (e.g. VWR 215-0917) |
||
|
416920SP |
Pipette Rack LV/MV (for Solvent, Sample, and DFE tips) |
2 |
|
414218SP |
Pipette Tip Waste Bin |
1 |
|
Biotage® VacMaster™-96 Processing & Accessories |
||
|
Part Number |
Description |
Qty |
|
121-9600 |
Biotage® VacMaster™-96 Sample Processing Manifold |
1 |
|
121-9602 |
Biotage® VacMaster™ VCU- 2 Vacuum Control and Generation Unit |
1 |
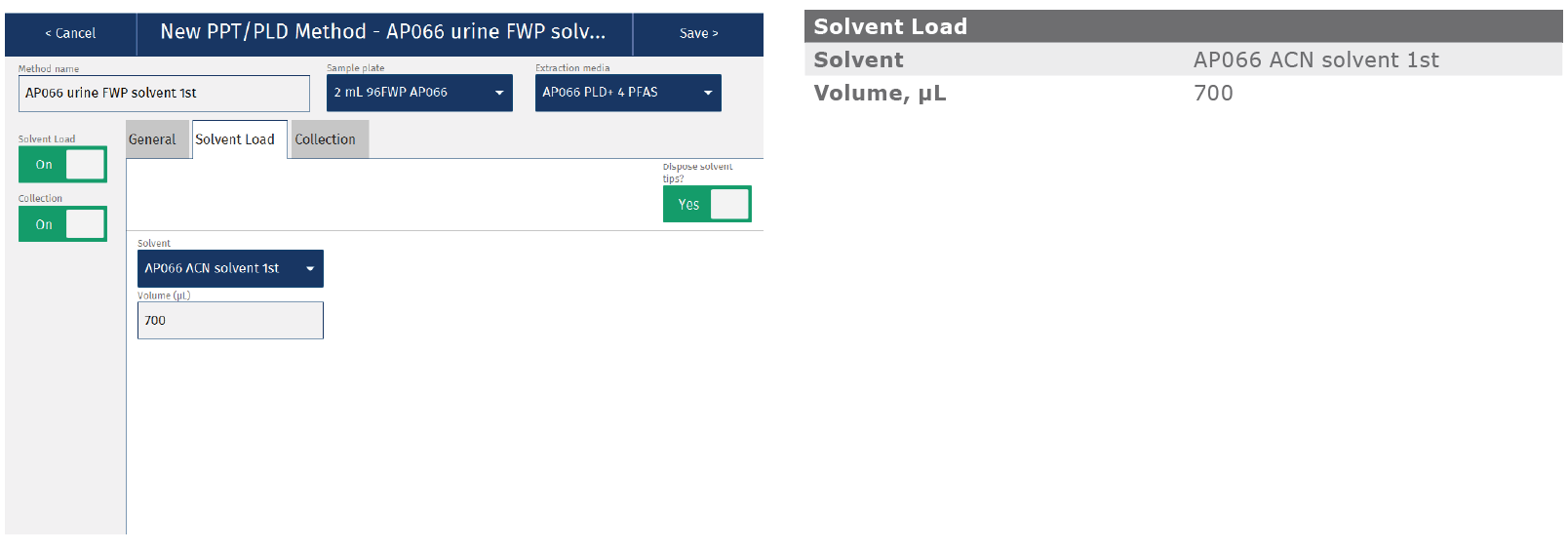
Appendix: Biotage® Extrahera™ LV-200 method
The method described in this application note was automated on the Biotage® Extrahera™ LV-200. This appendix contains the software settings required to configure the system to run this method for a 100 µL urine sample. Details for 50 µL sample are avail- able on request. As described in the main body of the application note, analyte recoveries, linearities and LOQs were comparable for both manually processed (Biotage® VacMaster™-96) and automated methods. Reproducibility was improved for samples extracted using the automated Extrahera™ LV-200 system. Total time for extraction of 96 samples using this method was 35 minutes.
|
Sample name |
AP066 urine solvent 1st |
|
Sample plate/rack |
2 mL 96FWP AP066 |
|
Extraction media |
AP066 PLD+ 4 PFAS |
|
Extraction solvent |
AP066 ACN solvent 1st |
Screen Shot Settings
Literature Number: AN994

