Automated extraction of THC and its metabolites from human whole blood with ISOLUTE® HAX using Biotage® Extrahera™ HV-5000
By Biotage
For research use only. NOT for use in diagnostic procedures.
Figure 1. Structures of analytes and internal standard.
Introduction
This application note describes the extraction of tetrahydrocannabinol (THC) and its metabolites from human whole blood using ISOLUTE® HAX SPE cartridges and Biotage® Extrahera™ HV-5000 prior to GC/MS analysis.
The simple sample preparation procedure, based on a mixed- mode/strong anion exchange extraction mechanism, delivers clean extracts and analyte recoveries greater than 70% with RSDs lower than 10%. Linearity of greater than 0.99 is achieved from 1–100 ng/mL.
This application note includes optimized conditions for automated processing of the HAX cartridges (using Biotage® Extrahera™ HV-5000) and manual processing (using the Biotage® PRESSURE+ 48 positive pressure manifold). Data generated using both processing systems is shown.
Analytes
Δ8-Tetrahydrocannabinol
Δ9-Tetrahydrocannabinol
11-Hydroxy-Δ-9- tetrahydrocannabinol
11-Nor-9-carboxy-Δ-9-tetrahydrocannabinol
Internal standard
11-Nor-9-carboxy-Δ-9-tetrahydrocannabinol-D3
Sample preparation procedure
Format:
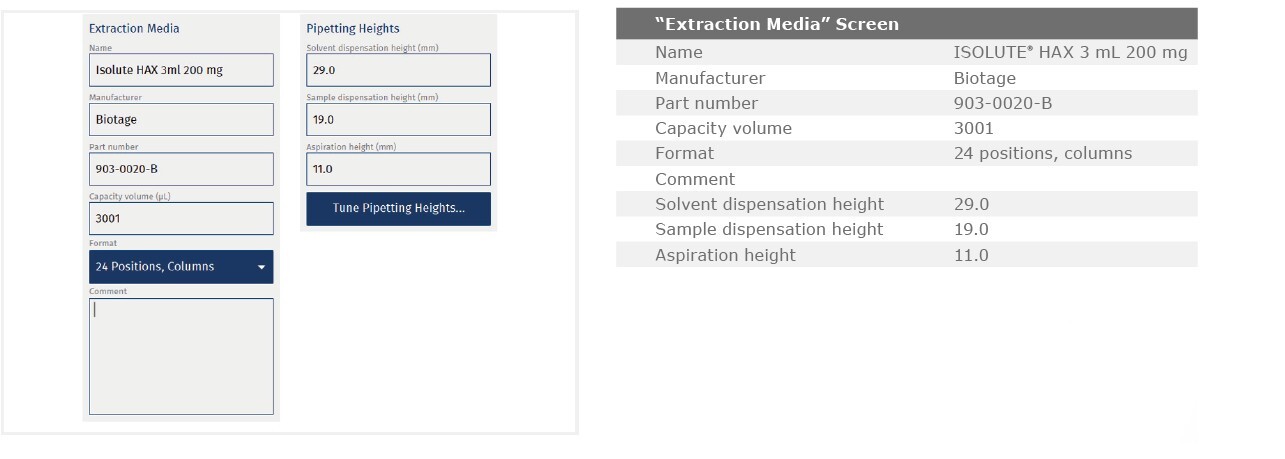
ISOLUTE® HAX 200 mg/3 mL cartridge, p/n 903-0020-B
Automated and manual processing:
Samples were processed manually using a Biotage® PRESSURE+ 48 positive pressure manifold. Each step below was processed at 2 to 4 psi using the adjustable flow setting. Drying steps were processed at 40 psi using the maximum flow setting.
Automated sample processing was performed using the Biotage® Extrahera™ HV-5000 system. Detailed processing conditions are included in the appendix.
Sample pre-treatment:
Spike whole blood (500 µL) with internal standard solution, add 500 µL ice cold acetonitrile dropwise and mix thoroughly. Centrifuge for 10 minutes at 12,000 RCF, take supernatant and add 1 mL H2O.
Note: Internal standard solution consists of a 10 ng/µL methanolic solution. 3 µL of this was added to 500 µL of whole blood to give a 60 ng/mL spike concentration.
Conditioning:
Condition with methanol (3 mL)
Equilibration:
Equilibrate with DI water (3 mL)
Sample loading:
Load the full volume of the pre-treated whole blood sample
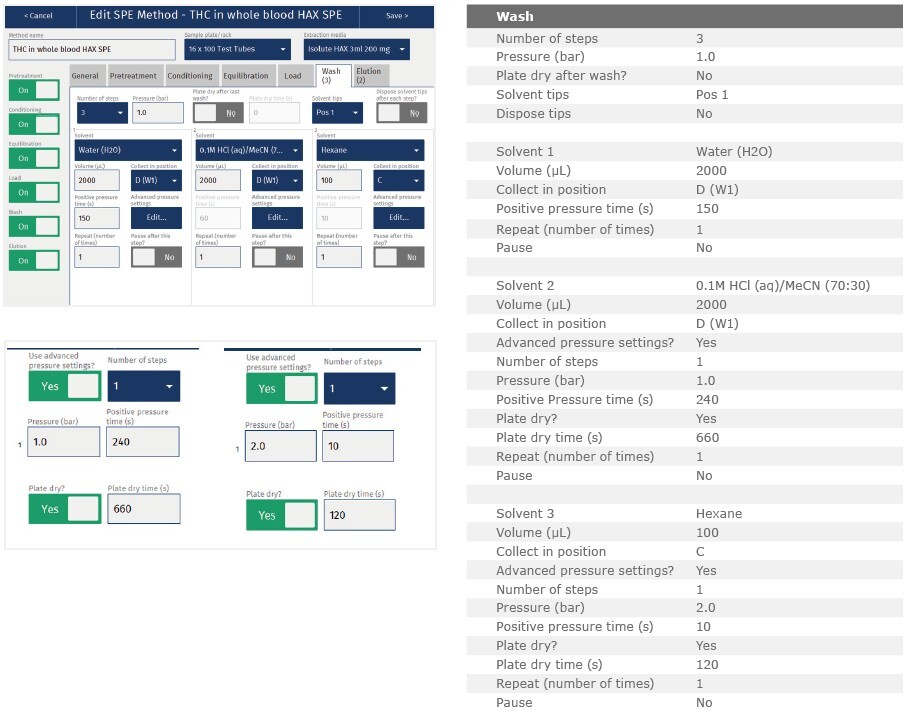
Wash 1:
Elute interferences with DI water (2 mL)
Wash 2:
Elute interferences with 100mM HCl (aq):ACN (70:30, v/v, 2 mL)
Dry:
Dry columns for 10 mins under positive pressure (40 psi)
Wash 3:
Elute interferences with hexane (100 µL)
Dry:
Dry columns for 10 mins under positive pressure (40 psi)
Wash 3:
Elute interferences with hexane (100 µL)
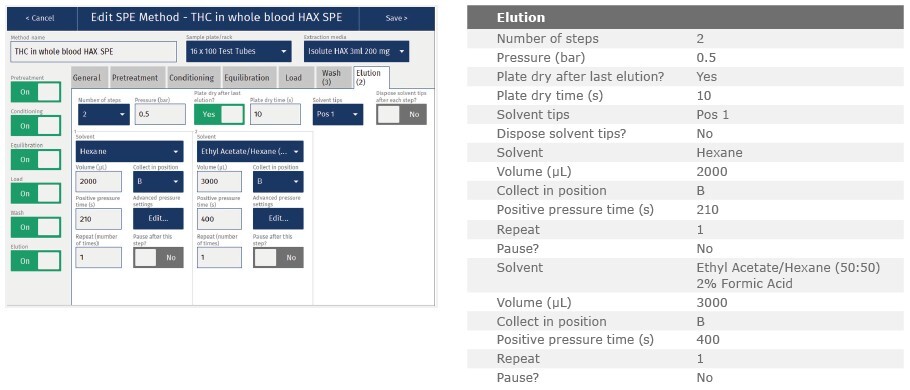
Elution 1:
Elute analytes with hexane (2 mL)
Elution 2:
Elute analytes with hexane:ethyl acetate (50:50, v/v,) with 2% formic acid (3 mL).
As THC-OH and THC-COOH are polar compounds, evaporation and derivatisation steps were necessary in this application in order to obtain a stabilised analyte that could be analysed via GC/MS.
Post elution & Reconstitution:
Transfer elution solvent tubes to the evaporation system. Dry the extract in a stream of air using a TurboVap® LV at 40 °C, 0.5 L/min for 10 minutes, followed by 1.0 L/min for 10 minutes.
Reconstitute evaporated samples with ethyl acetate (20 µL) and BSTFA:TMCS (99:1, 20 µL). Vortex mix, transfer to high recovery GC/MS vial and derivatise on a heat block at 70 °C for 25 minutes. Transfer to GC-MS for analysis.
GC conditions
Instrument:
Agilent 7890A GC with purged Ultimate Union
Column:
Agilent J&W DB-5ms 30 m, 0.25 mm, 0.25 µm
Mobile phase:
GC/MS grade helium
Inlet temperature:
260 °C Splitless
Flow rate:
1.5 mL/min
Injection volume:
1.5 μL
Table 1. GC oven gradient.
|
Ramp rate (°C/min) |
Temp (°C) |
Hold Time |
|
|
60 |
0 |
|
25 |
325 |
2 |
MS conditions
Instrument:
Agilent 5975C
Transfer line temp:
280 °C
Source temp:
230 °C
Quad temp:
150 °C
Table 2. MS conditions for target analytes in positive mode.
|
Analytes |
Quant ions |
Qual ions |
|
Δ 9 THC |
386 |
371, 303 |
|
Δ 8 THC |
303 |
386, 371 |
|
THC-OH |
371 |
474, 459 |
|
THC-COOH |
371 |
488, 473 |
|
THC-COOH-D3 |
374 |
476, 491 |
Results
Recovery and reproducibility
High (> 70%) and reproducible (RSD < 10%) recoveries were achieved for all analytes using the method described in this application note using the Biotage ISOLUTE® HAX cartridge format processed using the Biotage® Extrahera™ HV-5000. Figure 2. Shows average analyte % recoveries from spiked samples (N=7) using the optimized ISOLUTE® HAX protocol described in this application note. Processing was carried out manually using Biotage® PRESSURE+48 or automated using Biotage® ExtraheraTM HV-5000.
Figure 2. Shows average analyte % recoveries from spiked samples (N=7) using the optimized ISOLUTE® HAX protocol described in this application note. Processing was carried out manually using Biotage® PRESSURE+48 or automated using Biotage® ExtraheraTM HV-5000. Figure 3. Shows analyte % RSD obtained from spiked samples (N=7) using the optimized ISOLUTE® HAX protocol described in this application note. Cartridges were processed offline using Biotage® PRESSURE+ 48 and using the Biotage® ExtraheraTM HV-5000.
Figure 3. Shows analyte % RSD obtained from spiked samples (N=7) using the optimized ISOLUTE® HAX protocol described in this application note. Cartridges were processed offline using Biotage® PRESSURE+ 48 and using the Biotage® ExtraheraTM HV-5000.
Linearity
Calibration curve performance was investigated from whole blood spiked between 1–100 ng/mL. Good linearity was observed delivering r2 values greater than 0.998. Tables 3 and 4. detail linearity performance for manual and automated extractions, respectively.
Table 3. Analyte calibration curve r2 for manual extraction.
|
Analyte |
r2 |
|
Δ 8 THC |
0.998 |
|
Δ 9 THC |
0.999 |
|
THC-OH |
0.999 |
|
THC-COOH |
0.998 |
Table 4. Analyte calibration curve r2 for automated extraction.
|
Analyte |
r2 |
|
Δ 8 THC |
0.998 |
|
Δ 9 THC |
0.999 |
|
THC-OH |
0.999 |
|
THC-COOH |
0.998 |
Calibration curves
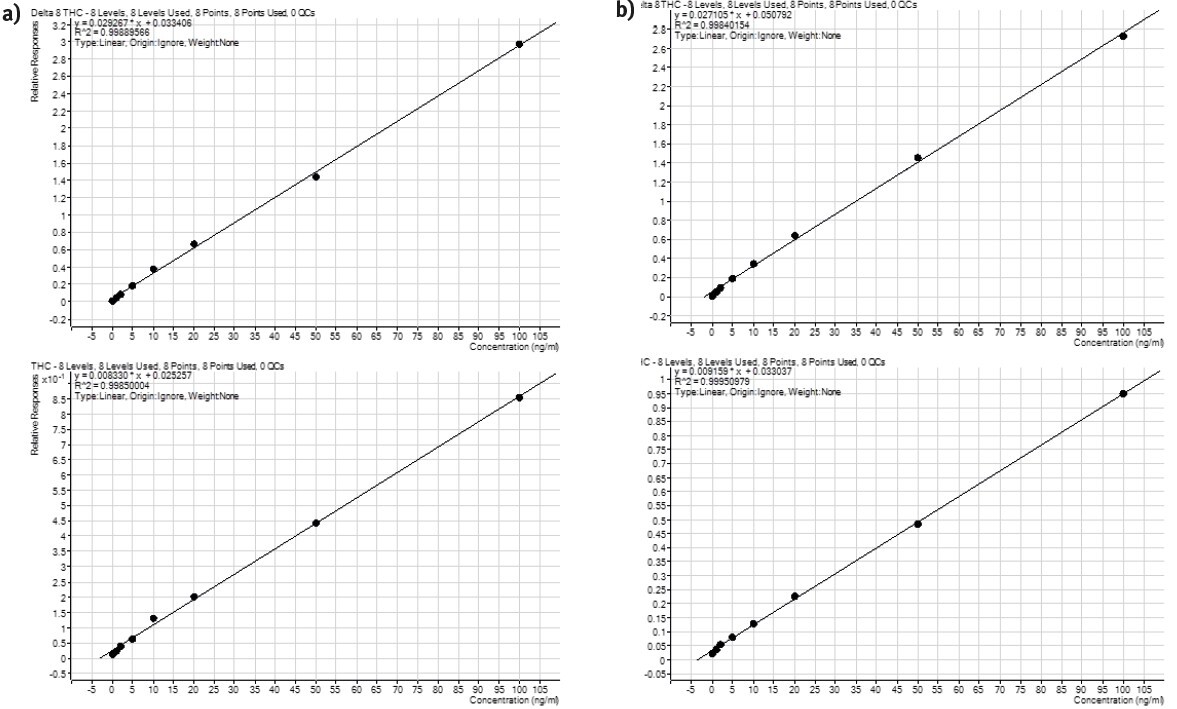
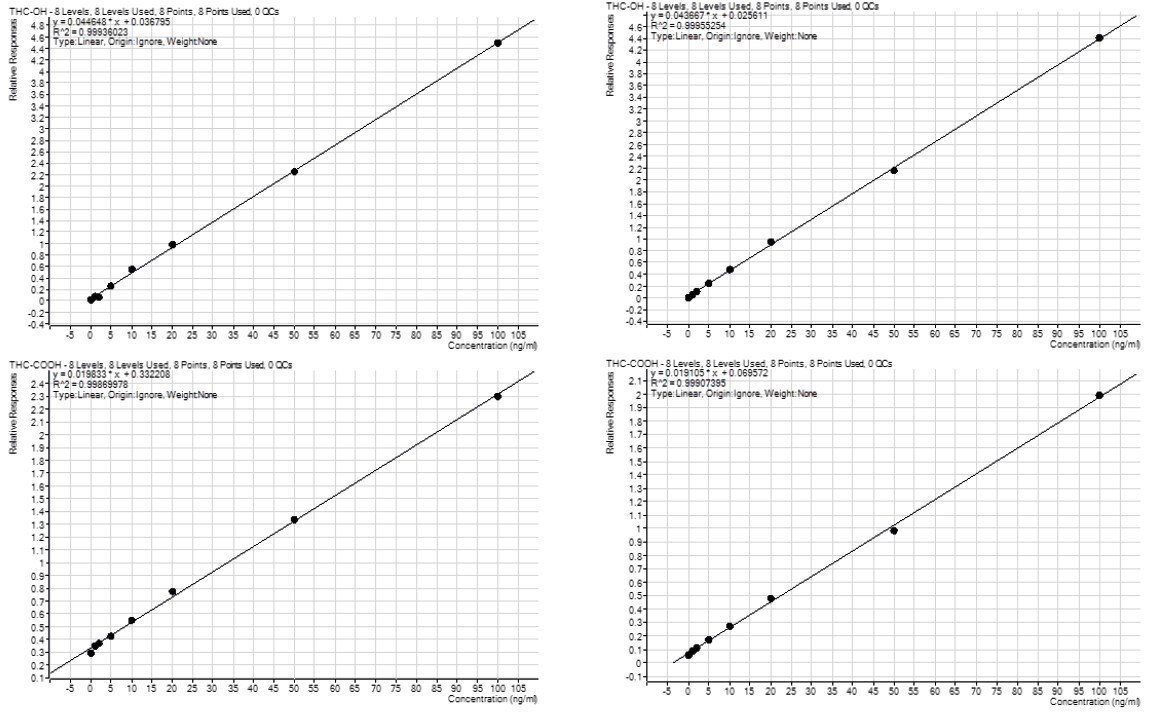
Figure 4. Calibration curves for THC, THC-OH and THC-COOH extraction on the Biotage® Extrahera™ HV-5000 (a) and PRESSURE+ 48 (b) using ISOLUTE® HAX cartridges to extract human blood samples.
Processing time
For a batch size of 24 samples, processing times were:
|
Processing System |
Processing Time |
|
Biotage® PRESSURE+ 48 |
60 minutes |
|
Biotage® Extrahera™ HV-5000 |
70 minutes |
Conclusion
ISOLUTE® HAX solid phase extraction elution cartridge provided robust manual or automated extraction of THC and its metabolites from pre-treated whole blood samples.
Good, reproducible recoveries were achieved, with an overall processing time of 70 minutes (excluding the common evaporation and derivatisation steps).
Chemicals and reagents
- Methanol (LC-MS grade), hexane, ethyl acetate and acetonitrile were purchased from Rathburn Chemicals Ltd (Walkerburn, UK).
- All analyte standards, deuterated internal standards, sodium hydroxide, hydrochloric acid, formic acid and acetic acid were purchased from Sigma- Aldrich Company Ltd. (Gillingham, UK).
- DI Water used was 18.2 MOhm-cm, drawn daily from a Direct-Q5 water purifier.
- Internal standard (10 ng/mL) was prepared from a 1 mg/mL stock solution by adding 10 µL to 990 µL of MeOH. 3 µL of this solution was then added to each calibration solution.
- Wash 2 solvent (100mM HCl (aq):ACN (70:30, v/v)) was made up by measuring out 70 mL of water (18.2 MOhm-cm) and 30 mL of acetonitrile and adding both to a bottle, then adding 833 µL of concentrated hydrochloric acid.
- Elution solvent (hexane:ethyl acetate (50:50, v/v with 2% formic acid)) was made up by measuring out 50 mL of hexane and 50 mL of ethyl accetate and adding both to a bottle with 2 mL formic acid.
Additional information
All data shown in this application note was generated using human whole blood donated by healthy human volunteers.
Ordering information
|
Part Number |
Description |
Quantity |
|
903-0020-B |
ISOLUTE® HAX cartridges, 200 mg/3mL |
50 |
|
PPM-48 |
Biotage® PRESSURE+ 48 Positive Pressure Manifold |
|
|
471002 |
Biotage® Extrahera™ HV-5000 |
1 |
|
417610 |
Configuration Kit 24 Positions Dual Flow - HV |
1 |
|
414174SP |
Column Rack 24 x 3 mL |
1 |
|
415491 |
Sample/Collection Rack 12 x 75 mm, 24 Positions |
1 |
|
414254SP |
Sample Rack 16 x 100 mm, 24 Positions |
1 |
|
415000 |
TurboVap® LV |
1 |
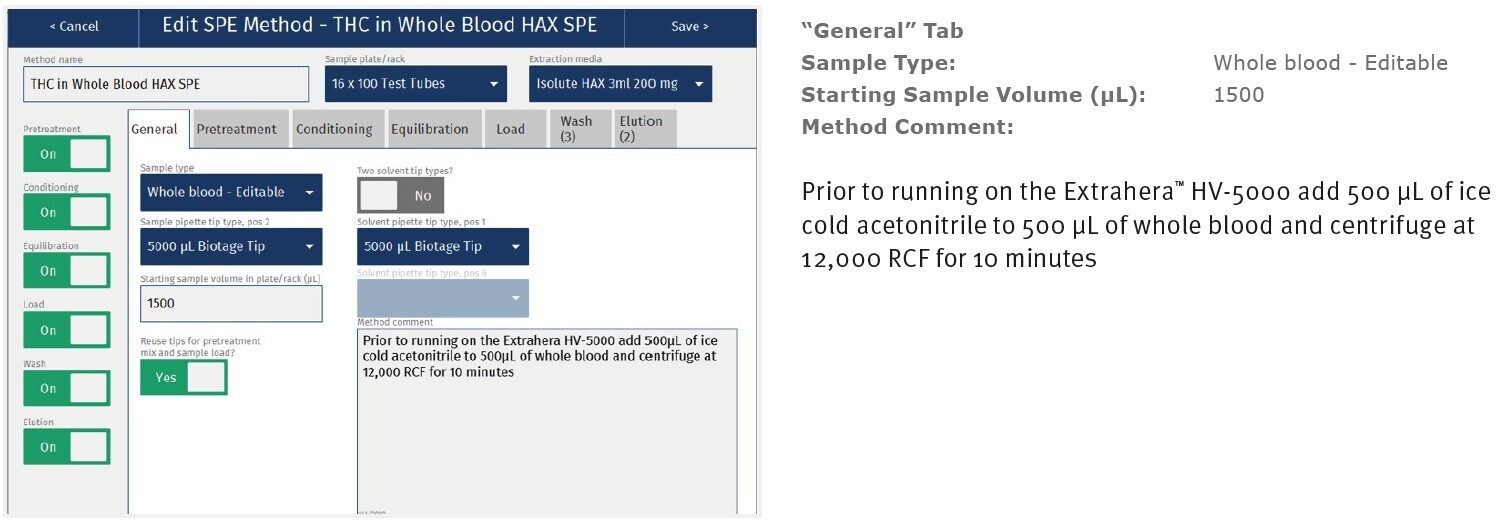
Appendix: Biotage® Extrahera™ HV-5000 settings
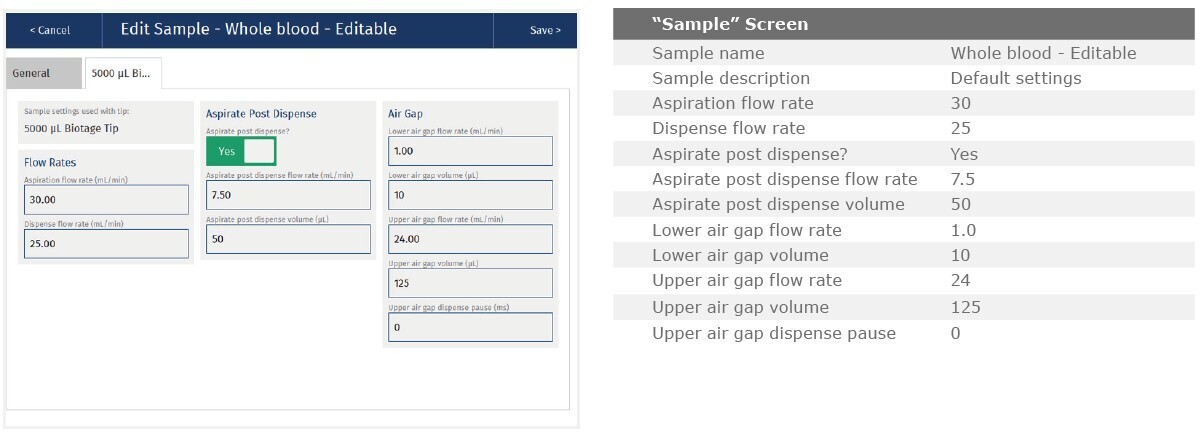
The method described in this application note was automated on the Biotage® Extrahera™ High Volume 5000 using ISOLUTE® HAX 200 mg/3 mL cartridges. This appendix contains the software settings required to configure Extrahera™ to run this method. Screenshots may or may not match those here depending upon the instrument software version.
|
Sample Name: |
THC in Whole Blood |
|
Sample Plate/Rack: |
16 x 100 test tubes |
|
Extraction Media: |
ISOLUTE® HAX 200 mg/3 mL |
Screenshot Settings
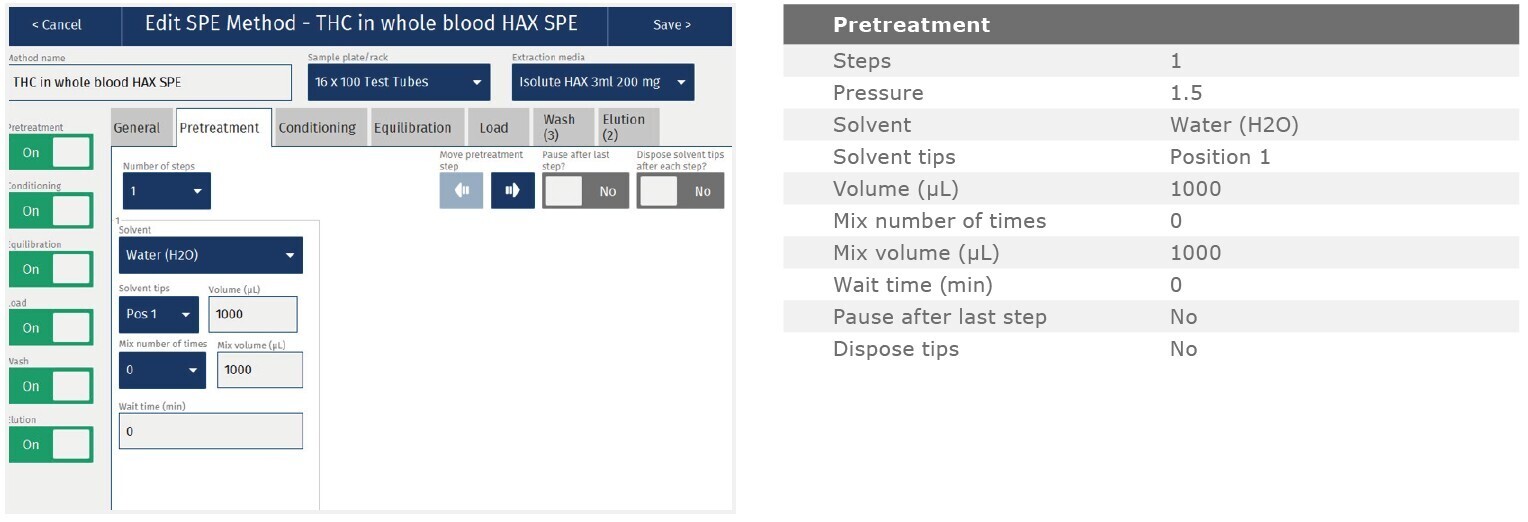
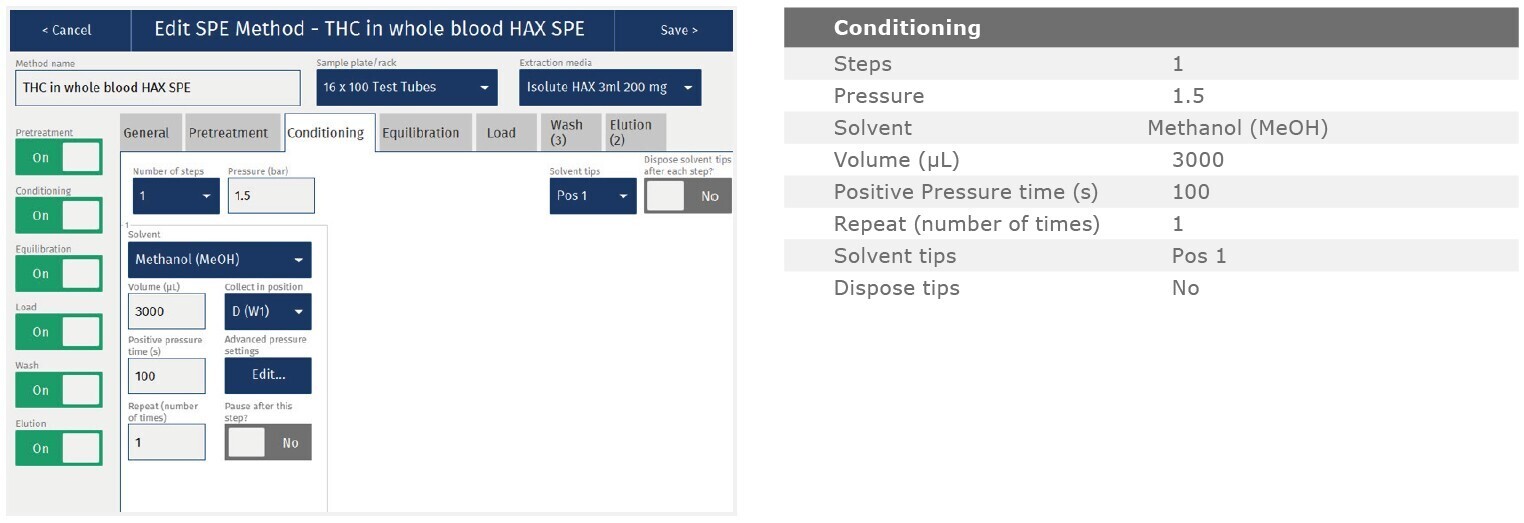
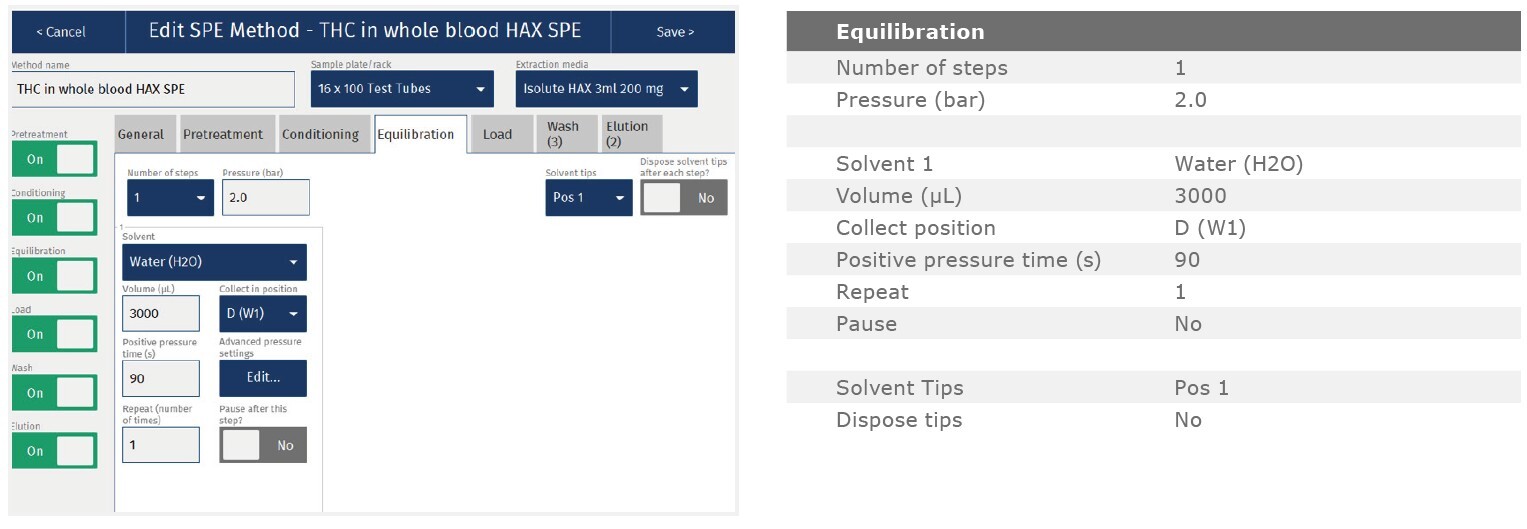
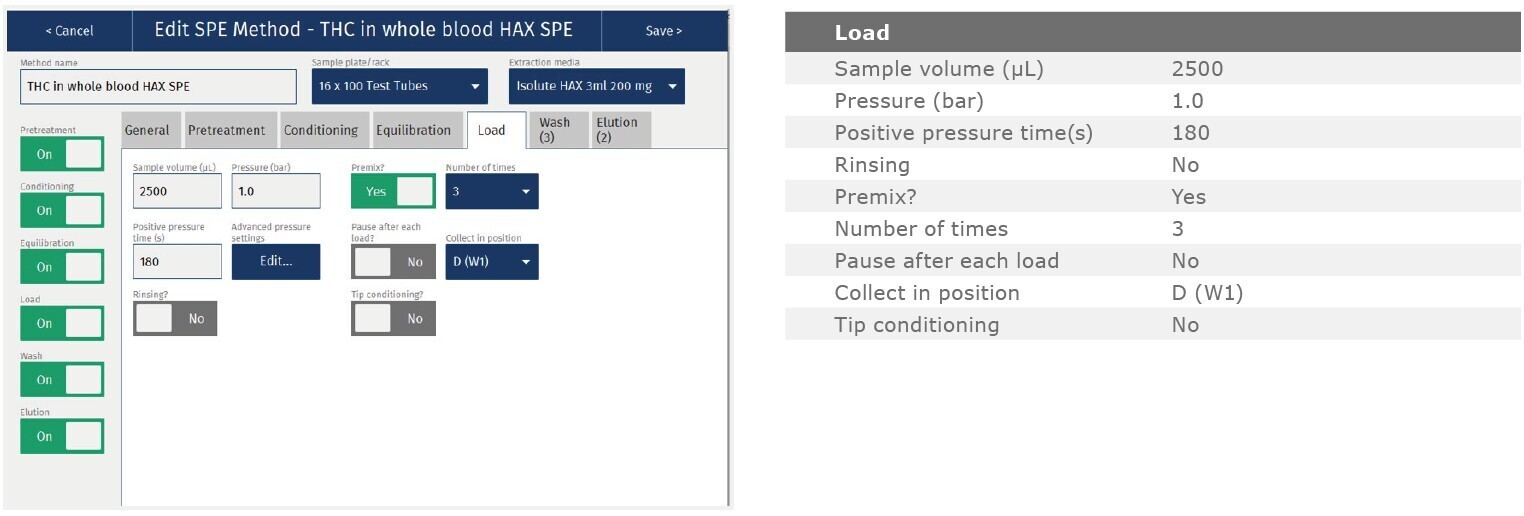
Solvent properties
|
Solvent Description |
|
|
1 |
Methanol |
|
2 |
Water (H2O) |
|
3 |
0.1M Sodium Acetate, pH3 (aq) |
|
4 |
0.1M HCl (aq)/MeCN (70:30) |
|
5 |
Ethyl Acetate/Hexane (50:50) 2% Formic Acid |
|
6 |
Hexane |
|
7 |
|
|
8 |
|
|
9 |
|
|
10 |
|
Solvent |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
|
Reservoir Type |
Refillable |
Non Refillable |
||||||||
|
Capacity |
N/A |
N/A |
N/A |
N/A |
N/A |
|||||
|
Aspiration flow rate |
50 |
50 |
50 |
50 |
50 |
50 |
||||
|
Dispense flow rate |
70 |
75 |
75 |
75 |
40 |
40 |
||||
|
Aspiration post dispense? |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
||||
|
Aspirate post dispense flow rate |
7.5 |
7.5 |
7.5 |
7.5 |
7.5 |
7.5 |
||||
|
Aspirate post dispense volume |
50 |
50 |
50 |
50 |
200 |
200 |
||||
|
Lower air gap flow rate |
1.0 |
1.0 |
1.0 |
1.0 |
1.0 |
1.0 |
||||
|
Lower air gap volume |
15 |
15 |
40 |
15 |
30 |
30 |
||||
|
Upper air gap flow rate |
10 |
24 |
24 |
10 |
5 |
5 |
||||
|
Upper air gap volume |
250 |
250 |
250 |
250 |
250 |
250 |
||||
|
Upper air gap dispense pause |
1000 |
1000 |
1000 |
1000 |
2000 |
2000 |
||||
|
Conditioning? |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
||||
|
Frequency |
1st Asp. only |
|||||||||
|
Cond. Times |
3 |
2 |
2 |
3 |
4 |
4 |
||||
|
Cond. Flow rate |
50 |
50 |
50 |
100 |
100 |
100 |
||||
|
Volume dependent conditioning |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
||||

Literature Number: AN971

