Extraction of PEth biomarkers in human whole blood using ISOLUTE® SLE+
By Biotage
For research use only. NOT for use in diagnostic procedures.
 Figure 1. Structures of PEth 16:0, Peth 16:0/18:1 and PEth 16:0/18:2.
Figure 1. Structures of PEth 16:0, Peth 16:0/18:1 and PEth 16:0/18:2.
Introduction
This application note describes the extraction of a panel of three phosphatidylethanol (PEth) alcohol biomarkers from human whole blood using ISOLUTE® SLE+ Supported Liquid Extraction plates prior to LC/MS-MS analysis. The simple sample preparation procedure delivers clean extracts and analyte recoveries greater than 60% with RSDs lower than 10% for all analytes, with reduced levels of phospholipids to improve extract cleanliness and analyte sensitivity. Linearity of greater than 0.999 is achieved for all analytes from 0.5–5000 ng/mL.ISOLUTE® SLE+ Supported Liquid Extraction plates offer an efficient alternative to traditional liquid-liquid extraction (LLE) for bioanalytical sample preparation, providing high analyte recoveries, no emulsion formation, and significantly reduced sample preparation.
Analytes
Phosphatidylethanol 16:0, Phosphatidylethanol 16:0/18:1 and Phosphatidylethanol 16:0/18:2
Internal standards
Phosphatidylethanol 16:0/18:1 – D5
Sample preparation procedure
Format
ISOLUTE® SLE+ 400 µL Sample Capacity Plate, Part Number 820-0400-P01
Sample pre-treatment
Add 10 µL of a 250 pg/µL ISTD solution (in H2O:isopropanol, 1:1, v/v) to 100 µL of human whole blood (to give a final concentration of 25 ng/mL).
Add a further 100 µL of H2O:MeCN (80:20 v/v). Mix thoroughly.
Sample loading
Load 200 µL of pretreated whole blood onto each ISOLUTE® SLE+ well. Ensure that the sample covers the entire area of the ISOLUTE SLE+ top frit. Using a Biotage® PRESSURE+96 Positive Pressure Manifold, apply a pulse of pressure to load samples onto the sorbent. Wait 5 minutes for the sample to equilibrate on the sorbent.
Analyte extraction
Apply an aliquot of ethyl acetate:isopropanol (95:5, v/v, 700 µL) and allow to flow under gravity for 5 minutes. Apply a second aliquot of ethyl acetate: acetate:isopropanol (95:5, v/v, 700 µL) and allow to flow under gravity for 5 minutes. Apply a final aliquot of ethyl acetate:isopropanol (95:5, v/v, 700 µL). For complete solvent recovery apply a pulse of positive pressure at 10 psi (10–20 seconds).
Collection vessels
Collect the eluent in 2 mL volume, 96-well collection plate.
Post elution
Evaporate extracts at 40 °C, for 30 mins at a flow rate of 20–40 L/min using the Biotage® SPE Dry-96.
Reconstitute
Reconstitute in isopropanol (200 µL). Vortex mix. Cover plate with a sealing mat prior to injection.
UHPLC conditions
Instrument
Shimadzu Nexera x 2 UHPLC
Column
Agilent Poroshell 120, EC-C8, 2.7 µm 2.1 x 50 mm with a guard column of the same chemistry
Mobile phase
A: 5 mM ammonium formate (aq) B: Isopropanol
Flow rate
0.3 mL/min
Column temperature
40 °C
Injection volume
5 µL
Table 1. UPLC Gradient.
|
Time (min) |
%A |
%B |
|
0 |
40 |
60 |
|
1 |
0 |
100 |
|
4 |
0 |
100 |
|
4.01 |
40 |
60 |
|
5.5 |
40 |
60 |
Mass spectrometry conditions
Equipment
Shimadzu 8060 Triple Quadrupole MS using ES interface
Nebulizing gas flow
3 L/min
Drying gas flow
5 mL/min
Heating gas flow
15 L/min
Interface temperature
400 oC
DL temperature
300 oC
Heat block temperature
500 oC
CID gas
270 kPa
Table 2. MS conditions and retention times for target analytes in positive and negative mode.
|
Analytes MRM T |
ransition |
Collision Energy |
Ion Mode |
|
PEth 16:0 |
675.20 255.25 |
32 |
|
|
(675.20 437.20) |
25 |
||
|
PEth 16:0/18:1 |
701.40 281.35 |
34 - |
|
|
(701.40 437.30) |
24 |
||
|
PEth 16:0/18:2 |
699.40 279.30 |
32 - |
|
|
(699.40 255.30) |
35 |
Results
This simple sample preparation method delivers clean extracts and analyte recoveries mostly greater than 60% with RSDs lower than 10% for all analytes (see Figure 2), and LLOQs below 10 ng/mL for all 3 phosphatidylethanol species. Figure 2. below shows recoveries obtained using 400 µL capacity ISOLUTE® SLE+ plates, when loading 200 µL of pre-treated whole blood sample. Figure 2. Typical analyte % extraction recoveries (n=7) loading 200 µL of pre-treated whole blood.
Figure 2. Typical analyte % extraction recoveries (n=7) loading 200 µL of pre-treated whole blood.
Calibration curve performance was investigated from human whole blood spiked between 0.5–5000 ng/mL. Good linearity was observed for all analytes typically delivering r2 values greater than 0.999. Table 3. details linearity performance and associated LOQ for each analyte loading 200 µL of pre-treated whole blood onto a 400 µL capacity plate. Calibration curves for all analytes are shown in Figure 3.
Table 3. Analyte calibration curve r2 and LOQ performance.
|
Analyte |
r2 |
LLOQ (ng/mL) |
|
PEth 16:0 |
0.9994 |
0.5 |
|
PEth 16:0/18:1 |
0.9993 |
0.5 |
|
PEth 16:0/18:2 |
0.9994 |
5 |
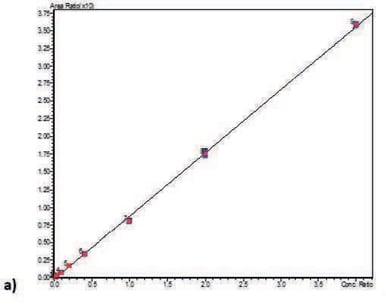
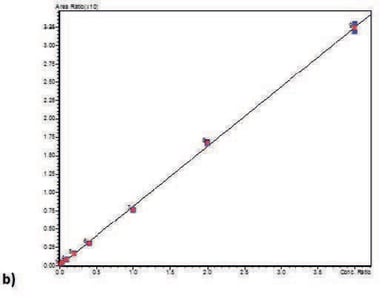
 Figure 3. Calibration curves for PEth 16:0 (a), PEth 16:0/18:1 (b), and PEth 16:0/18:2 (c) in whole blood.
Figure 3. Calibration curves for PEth 16:0 (a), PEth 16:0/18:1 (b), and PEth 16:0/18:2 (c) in whole blood.
Discussion
When analyzing blood products, it is important to eliminate interferences caused by phospholipids. Unfortunately, phosphatidylethanol is very similar in structure to phospho- lipids that can reduce sensitivity and quickly lead to the degradation of the LC column and MS system. One of the main aims of this study was to develop a sample preparation method that would allow for good recovery of the phosphatidylethanol species without significant levels of phospholipids being present in the extract. The optimum extraction solvent system identified to achieve this leads to slightly reduced recovery of the three phosphatidylethanol species, however the method sensitivity is such that the required detection limits are exceeded.
In this extraction, underloading of the sample was utilized to further clean up the extraction of phospholipids. Underloading (i.e. loading a lower sample volume than the theoretical capacity of the extraction product) when using whole blood also helps to eliminate possible breakthrough of red blood cells, as well as allowing for the use of more polar elution solvents.
Conclusion
This method provides clean extracts and highly sensitive detection for the analysis of three phosphatidylethanol biomarkers. This method has LOQs of at least 5 ng/mL with the usual cut off being 20 ng/mL.
Ordering information
|
Part Number |
Description |
Quantity |
|
820-0400-P01 |
ISOLUTE® SLE+ 400 µL 96-Well Plate |
1 |
|
PPM-96 |
Biotage® PRESSURE+ 96 Positive Pressure Manifold |
1 |
|
SD-9600-DHS-EU |
Biotage® SPE Dry 96 Sample Evaporator 220/240 V |
1 |
|
SD-9600-DHS-NA |
Biotage® SPE Dry 96 Sample Evaporator 100/120 V |
1 |
|
121-5203 |
Collection Plate, 2 mL Square |
50 |
|
121-5204 |
Pierceable Sealing Mat |
50 |
Chemicals and reagents
- Acetonitrile (LC-MS grade), isopropanol (Gradient MS) and ethyl acetate were purchased from Honeywell Research Chemicals (Bucharest, Romania).
- All analyte standards, deuterated internal standards and ammonium formate were purchased from Sigma- Aldrich Company Ltd. (Gillingham, UK).
- Water used was 18.2 MOhm-cm, drawn daily from a Direct-Q5 water purifier.
- Mobile phase A (5 mM ammonium formate (aq)) was prepared by adding 315.3 mg of ammonium formate to 1 L purified water.
- Internal standards (250 pg/µL) were prepared from a 10 ng/µL stock solution by adding 25 µL of each of to 955 µL of H2O:isopropanol (50:50 v/v). 10 µL of this solution was then added to each calibration solution.
- Pre-treatment solution was prepared by adding 20 mL of MeCN to 80 mL of purified water.
- Elution solvent was prepared by mixing 95 mL ethyl acetate with 5 mL isopropanol.
Additional information
- All data shown in this application note was generated using human whole blood from Golden West (California, US) with LiHep anti-coagulant.
- For increased sensitivity:
- Decrease reconstitution solvent volume below 200 µL
- Decrease reconstitution solvent volume below 200 µL
Literature Number: AN934

