Extraction of veterinary growth promoters from animal urine using ISOLUTE® SLE+
By Biotage
Figure 1. Representative veterinary growth promoter chemical structures.
This application note describes the extraction of 18 growth promoters (steroids, resorcylic acid lactones and stilbenes) using ISOLUTE® SLE+ supported liquid extraction prior to LC/MS analysis.
ISOLUTE® SLE+ Supported Liquid Extraction cartridges offer an efficient alternative to traditional liquid liquid extraction (LLE) for bioanalytical sample preparation, providing high analyte recoveries, no emulsion formation and significantly reduced preparation time. This application note provides an effective and efficient ISOLUTE SLE+ procedure optimized for the 1 mL sample capacity cartridge format. This simple sample preparation delivers clean extracts and analyte recoveries greater than 80% for most analytes. RSDs are typically less than 10% for all analytes (15% or lower at LOQ). LLOQ are typically at or below the estimated linear range minima.
Analytes
ß-trenbolone, α-trenbolone, ß-boldenone, α-boldenone, ß-nortestosterone, α-nortestosterone, methylboldenone, methyltestosterone, 16-ß-hydroxystanozolol, taleranol, zeranol, ß-zearalenol, α zearalenol, zearalenone, zearalanone, diethyl- stilbestrol, dienestrol, hexestrol
Internal standards
N/A
Sample preparation procedure
Format
ISOLUTE® SLE+ 1 mL cartridges, part number 820-0140-C
ISOLUTE® SLE+ 1 mL cartridges (Tabless), part number 820-0140-CG
Matrix preparation
Hydrolyze 500 µL animal urine as follows: dilute 1:1 (v./v) with 200 mM ammonium acetate pH 5.2 containing 10 µL per mL of glucuronidase H-2 ex. Helix pomatia. Incubate at 60 °C for 2 hours. Cool to room temperature.
Sample loading
Load 750 µL of hydrolyzed sample directly to ISOLUTE® SLE+ sorbent and apply a pulse of pressure 3–5 seconds to initiate flow. Allow the sample to absorb for 5 minutes.
Analyte extraction
Apply ethyl acetate/hexane (70:30, v/v, 3 mL) allow to flow under gravity for 5 minutes. For complete removal apply a pulse of positive pressure at 10 psi (10–20 seconds).
Post elution and reconstitution
Evaporate the extract at 40 °C and reconstitute in 400 µL 0.1% formic acid in 50% aq MeOH.
UHPLC conditions
Instrument
Shimadzu Nexera UHPLC
Column
ACE UltraCore Super C18 2.5 µm 100 x 2.1 mm (CORE-25A-1002U), VWR International (Lutterworth, UK)
Mobile phase
A: 0.5 mM ammonium acetate, 0.01% acetic acid, 30% MeOH (aq) B: 0.5 mM ammonium acetate, 0.01% acetic acid, 95% MeOH (aq)
Flow rate
0.5 mL min-1
Injection volume
10 μL
Column temperature
45 °C
Table 1. Gradient and divert valve settings
|
Time (min) |
%A |
%B |
Divert Valve |
|
0.00 |
90.0 |
10.0 |
waste |
|
7.00 |
NA |
NA |
MS |
|
8.00 |
75.0 |
25.0 |
|
|
12.00 |
62.5 |
37.5 |
|
|
14.00 |
NA |
NA |
waste |
|
14.50 |
50.0 |
50.0 |
|
|
16.50 |
10.0 |
90.0 |
|
|
18.00 |
10.0 |
90.0 |
|
|
20.00 |
90.0 |
10.0 |
|
|
24.50 |
90.0 |
10.0 |
Table 2. Typical retention times.
|
Analyte |
Retention Time (min) |
|
taleranol |
7.5 |
|
β-trenbolone |
8.1 |
|
β-zearalenol |
8.4 |
|
β-boldenone |
9.1 |
|
α-trenbolone |
9.4 |
|
β-nortestosterone |
9.6 |
|
zeranol |
10.5 |
|
methylboldenone |
10.6 |
|
α-zearalenol |
11.2 |
|
zearalanone |
11.5 |
|
α-boldenone |
11.7 |
|
diethylstilbestrol |
11.9 |
|
zearalenone |
12.0 |
|
α-nortestosterone |
12.0 |
|
16-β-hydroxystanozolol |
12.1 |
|
dienestrol |
12.6 |
|
methyltestosterone |
12.9 |
|
hexestrol |
13.1 |
MS conditions
Instrument
Sciex Triplequad 5500 operating in dual polarity ESI mode
Source temperature (TEM)
600 oC
Curtain Gas (CUR)
35
Source Gas 1 (GS1)
60
Source Gas 1 (GS2)
50
Table 3. MS parameters for target analytes.
|
Analyte |
Transition (Da) |
IS (V) |
DP (V) |
EP (V) |
CE (V) |
CXP (V) |
|
taleranol |
321.2 > 277.1 |
-3000 |
-112 |
-4 |
-30 |
-43 |
|
β-trenbolone |
271.0 > 199.0 |
+4500 |
48 |
5 |
30 |
22 |
|
β-zearalenol |
319.3 > 159.9 |
-3000 |
-85 |
-4 |
-38 |
-18 |
|
β-boldenone |
287.2 > 135.0 |
+4500 |
73 |
9 |
21 |
18 |
|
α-trenbolone |
271.0 > 199.0 |
+4500 |
30 |
4.5 |
32 |
22 |
|
β-nortestosterone |
275.2 > 108.8 |
+4500 |
49 |
5.5 |
32 |
14 |
|
zeranol |
321.2 > 277.0 |
-3000 |
-100 |
-4 |
-30 |
-38 |
|
methylboldenone |
301.1 > 120.8 |
+4500 |
85 |
9.5 |
30 |
16.5 |
|
α-zearalenol |
319.2 > 275.0 |
-3000 |
-180 |
-4 |
-28 |
-44 |
|
zearalanone |
319.3 > 275.0 |
-3000 |
-90 |
-7 |
-28 |
-48 |
|
α-boldenone |
287.2 > 120.9 |
+4500 |
62 |
7.5 |
31 |
18 |
|
diethylstilbestrol |
267.1 > 237.0 |
-3000 |
-210 |
-8 |
-37 |
-48 |
|
zearalenone |
317.2 > 131.0 |
-3000 |
-110 |
-4 |
-32 |
-17 |
|
α-nortestosterone |
275.0 > 108.8 |
+4500 |
20 |
4.5 |
33 |
14 |
|
16-β-hydroxystanozolol |
345.4 > 94.90 |
+4500 |
70 |
5 |
51 |
13 |
|
dienestrol |
265.0 > 92.80 |
-3000 |
-130 |
-4 |
-31 |
-12 |
|
methyltestosterone |
303.2 > 97.00 |
+4500 |
82 |
8 |
31 |
17 |
|
hexestrol |
269.1 > 134.0 |
-3000 |
-74 |
-4 |
-20 |
-17 |
Results
Recovery
Extraction recoveries were determined at 1 ng mL-1, equivalent to 400 pg when extracting 750 µL of pre treated hydrolyzed urine from four different species: bovine, equine, ovine and porcine.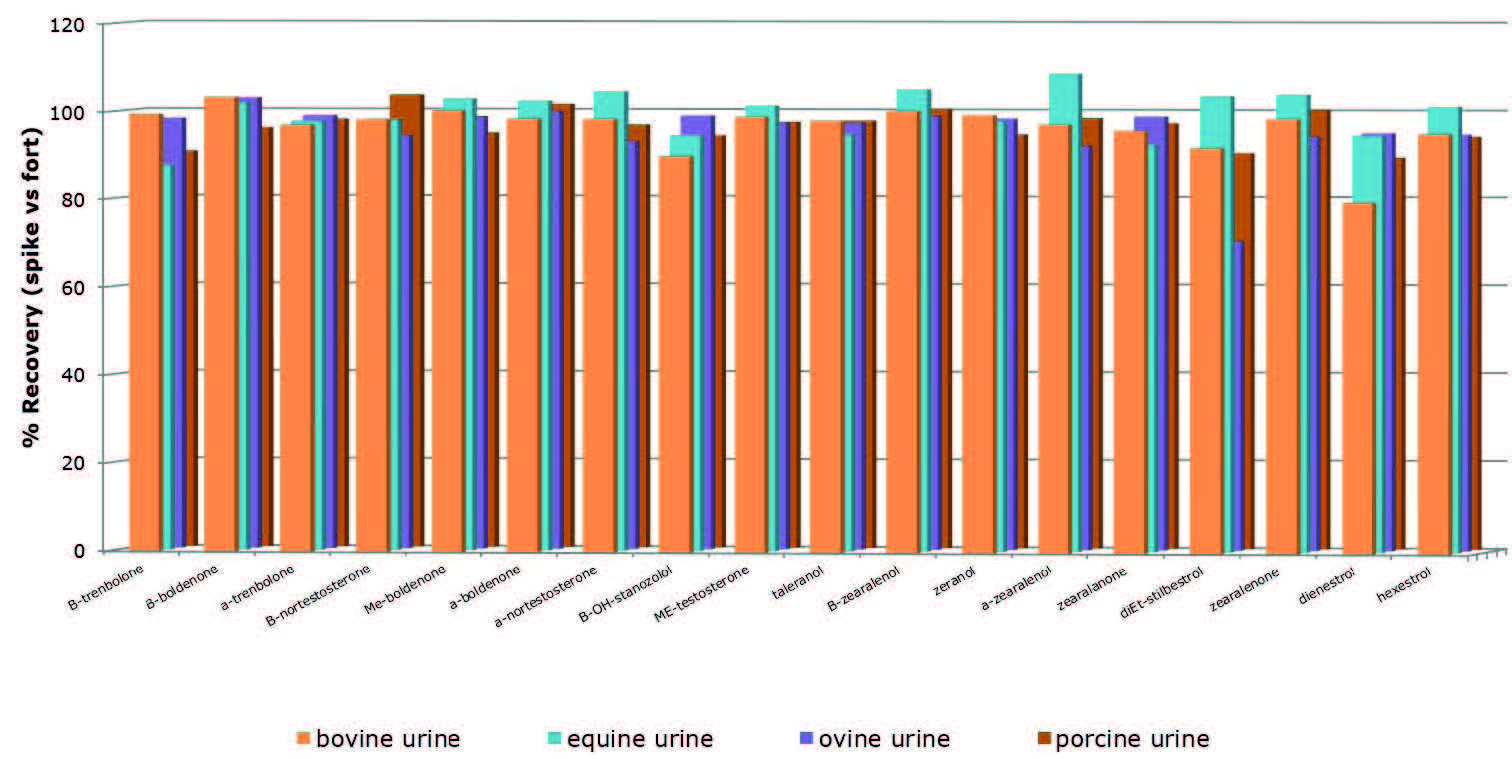
Figure 2. Representative analyte recoveries using an optimized ISOLUTE® SLE+ protocol (1 ng mL-1 spike, 375 µL of urine extracted).
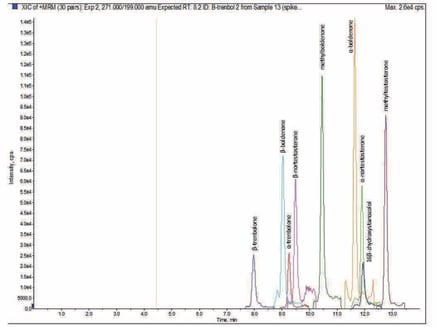
Figure 3. Representative +ESI target analyte chromatography (1 ng mL-1 spike).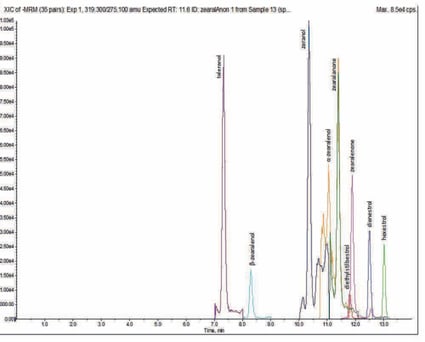
Figure 4. Representative -ESI target analyte chromatography (1 ng mL-1 spike).
Linearity
Extraction linearity was determined between 0.2 and 10 ng mL-1 from a mixed stock spiked into bovine urine and serially diluting in bovine urine. Each calibration level was extracted in duplicate. Table 4 details linearity performance and associated LOQ for each analyte. Calibration levels were accepted at 90–110% recovery with RSD <10% (lowest standard 80–120%, <20% RSD). Figure 5 demonstrates representative calibration curves, good linearity was observed for all analytes typically delivering r² values greater than 0.999. Zearalanone, a-zearalenol and diethylstilbestrol demonstrated a reduced linear range compared with other analytes.
Table 4. Analyte calibration curve r2 and LOQ performance.
|
Analyte |
Coefficient (r²) |
Linear range, ng mL-1 |
LOQ, ng mL-1 |
% accuracy (RSD) at LOQ |
|
taleranol |
0.9998 |
0.5 to 10 |
0.5 |
101.8 (2.2) |
|
β-trenbolone |
0.9998 |
0.2 to 10 |
0.2 |
82.7 (10.6) |
|
β-zearalenol |
0.9994 |
0.2 to 10 |
0.2 |
103.5 (0.5) |
|
β-boldenone |
0.9998 |
0.2 to 10 |
0.2 |
92.8 (0.6) |
|
α-trenbolone |
0.9998 |
0.2 to 10 |
0.2 |
91.0 (1.7) |
|
β-nortestosterone |
0.9996 |
0.2 to 10 |
0.2 |
94.3 (6.9) |
|
zeranol |
0.9996 |
0.2 to 10 |
0.2 |
93.1 (6.9) |
|
methylboldenone |
0.9996 |
0.5 to 10 |
0.5 |
95.0 (4.8) |
|
α-zearalenol |
0.9986 |
1.0 to 10 |
1.0 |
106.8 (3.6) |
|
zearalanone |
0.9954 |
0.2 to 1.0 |
0.2 |
96.3 (0.5) |
|
α-boldenone |
0.9998 |
0.2 to 10 |
0.2 |
90.7 (13.4) |
|
diethylstilbestrol |
0.9998 |
0.2 to 5.0 |
0.2 |
104.8 (12.7) |
|
zearalenone |
0.9984 |
0.2 to 10 |
0.2 |
102.2 (0.3) |
|
α-nortestosterone |
0.9996 |
0.5 to 10 |
0.5 |
99.4 (1.3) |
|
16-β-hydroxystanozolol |
0.9996 |
0.2 to 10 |
0.2 |
85.5 (9.5) |
|
dienestrol |
0.9988 |
0.5 to 10 |
0.5 |
105.2 (0.3) |
|
methyltestosterone |
0.9996 |
0.2 to 10 |
0.2 |
81.2 (6.1) |
|
hexestrol |
0.9996 |
0.2 to 10 |
0.2 |
101.4 (2.9) |
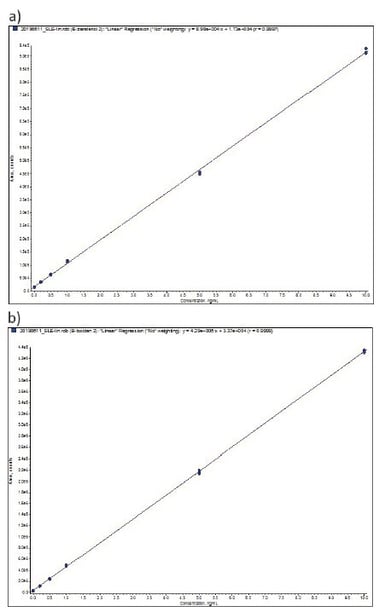
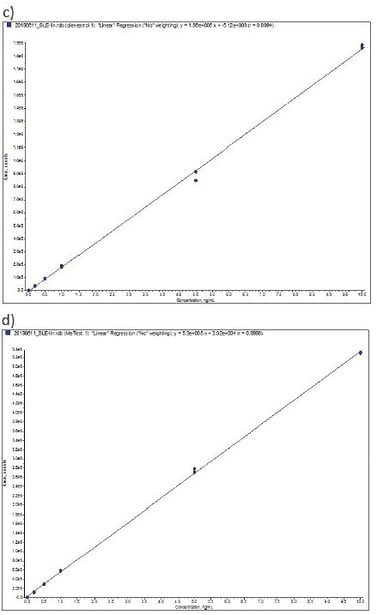
Figure 5. Representative Calibration curves (0.2 to 10 ng mL-1 extracted from bovine urine) a) β-zearalenol, b) β-boldenone, c) dienestrol, d) methyltestosterone.
Chemicals and reagents
Water (18.2 MΩ.cm) was drawn fresh daily from a Millipore Direct-Q5 water purifier (Watford, UK).
Organic solvents (methanol, ethyl acetate and hexane) were HPLC or LC-MS grade and purchased from Honeywell Research Chemicals (Bucharest, Romania).
Acetic acid (LC-MS) was purchased from Fisher Scientific UK (Loughborough, UK).
Ammonium acetate (reagent and LC-MS grade), formic acid (LC-MS grade) and ß-glucuronidase (Type HP-2, aqueous solution ≥100,000 units/mL) were purchased from Sigma- Aldrich Company Ltd. (Gillingham, UK).
Analytical standards were purchased from Sigma-Aldrich Company Ltd. (Gillingham, UK) or Toronto Research Chemicals (Toronto, Canada)
200 mM ammonium acetate pH 5.2 (aq) was prepared by diluting 15.42 g of reagent grade ammonium acetate in 1 L deionised water, the pH was adjusted using concentrated formic acid.
Buffered ß-glucuronidase hydrolysis solution was prepared fresh daily by diluting 500 µL ß-glucuronidase solution in 49.5 mL 200 mM ammonium acetate pH 5.2 (aq) and vortex- mixing thoroughly.
Ethyl acetate/hexane (70:30, v/v) was prepared by adding 30 mL hexane to 70 mL ethyl acetate and mixing thoroughly by inversion.
50% methanol (aq), 0.1% formic acid (reconstitution solution) was prepared by adding 50 mL methanol to 50 mL deionized water, followed by 100 µL concentrated LC-MS grade formic acid.
Mobile phase A (0.5 mM ammonium acetate (aq), 0.01 % acetic acid) was prepared by diluting 38.5 mg LC-MS grade ammonium acetate in 500 mL deionized water, adding 100 µL of concen- trated acetic acid and making up to 1 L with deionized water.
Mobile phase B (0.5 mM ammonium acetate (methanol), 0.01 % acetic acid) was prepared by diluting 38.5 mg LC-MS grade ammonium acetate in 500 mL LC-MS grade methanol, adding 100 µL of concentrated acetic acid and making up to 1 L with LC-MS grade methanol.
Additional information
Animal urine matrix samples were kindly donated by the State Laboratory, Celbridge, Ireland.
Ordering information
|
Part Number |
Description |
Quantity |
|
820-0140-C |
ISOLUTE® SLE+ 1 mL Cartridges |
30 |
|
820-0140-CG |
ISOLUTE® SLE+ 1 mL Cartridges (Tabless) |
30 |
|
PPM-48 |
Biotage® PRESSURE+ 48 Positive Pressure Manifold |
1 |
|
415000 |
TurboVap® LV |
1 |
Literature Number: AN924

