Sample preparation method for determination of carbapenems in plasma using ISOLUTE® ENV+
By Biotage

Figure 1. Structural formulae of carbapenem antimicrobials.
Introduction
Meropenem and Doripenem (Figure 1) are carbapenem antimicrobials that inhibit bacterial cell-wall synthesis and have bactericidal effects. The range of antibacterial activity is very broad and they show antibacterial action against a variety of bacteria. Therapeutic Drug Monitoring (TDM), which measures blood levels of the antibacterial drugs and helps design dosages for individual patient administration, is critical for the effective and safe use of the drugs.
In this application note, ISOLUTE® ENV+ (based on a hydroxyl- ated polystyrene divinylbenzene copolymer) (Figure 2) was used as a sample preparation column. It is suitable for retention of highly polar compounds that are typically not retained by silica-based C18 columns. In this application, Meropenem and Doripenem could be extracted from plasma samples with high recoveries.
Analytes
Meropenem (CAS: 119478-56-7)
Doripenem (CAS: 148016-81-3)
Sample preparation procedure
Format
ISOLUTE® ENV+ 25 mg/ 1 mL columns, (p/n: 915-0002-A)
An equivalent 96-well plate format (p/n: 915-0040-P01) is available.
Sample pre-treatment
To plasma (200 μL), add 0.1 μg/mL meropenem or doripenem as an internal standard. Add water (HPLC grade or higher) (200 μL) and vortex mix for 30 seconds.
Conditioning
Condition the column with methanol (1 mL)
Equilibration
Equilibrate the column with water (1 mL)
Sample loading
Load 400 μL of the pre-treated sample
Wash
Elute interferences with 10 mmol/L ammonium formate solution (1 mL)
Elution
First, elute with 10 mmol/L aqueous ammonium formate: methanol (8:2, (v/v) 0.5 mL). Next, elute with 0.5 mL of methanol. Combine the two eluates to obtain sample for LC-MS/MS.
Dilution
Depending on the range of calibration concentration in LC-MS/MS to be used, dilute the sample solution further*.
* In this application note, doripenem samples were diluted 2-fold using purified water.
UHPLC conditions
Instrument
Shimadzu Nexera LC-30AD
Column
Waters ACQUITY UPLC BEH C18 1.7 μm (2.1 mm × 50 mm column)
Mobile phase
A: 10 mmol/L aqueous ammonium formate with 0.1% formic acid
B: Methanol with 0.1% formic acid
Flow rate
0.4 mL/min.
Table 1. Gradient Conditions.
|
Time (min) |
%B |
|---|---|
|
0 |
5 |
|
0.5 |
5 |
|
2 |
50 |
|
2.1 |
90 |
|
2.5 |
90 |
|
2.51 |
5 |
|
3.5 |
5 |
Column Temperature
50 °C
Injection volume
1 µL
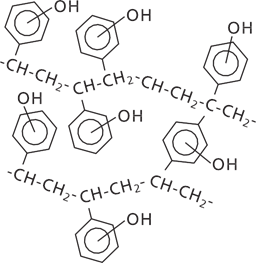
Figure 2. Schematic representation of ISOLUTE® ENV+
Mass spectrometry conditions
Equipment
Shimadzu LCMS-8060
Ionization mode
ESI positive
Nebulizer gas flow rate
3.00 L/mim
Drying gas flow rate
10.00 L/min
Heating gas flow rate
10.00 L/min
Interface temperature
350 °C
DL temperature
200 °C
Heat block temperature:
350 °C
CID gas
270 kPa
SRM transitions
Meropenem: m/z 384.00 > 141.15, Rt 1.82 min, Collision Energy;-16
Doripenem: m/z 420.90 > 274.00, Rt 1.56 min, Collision Energy;-16
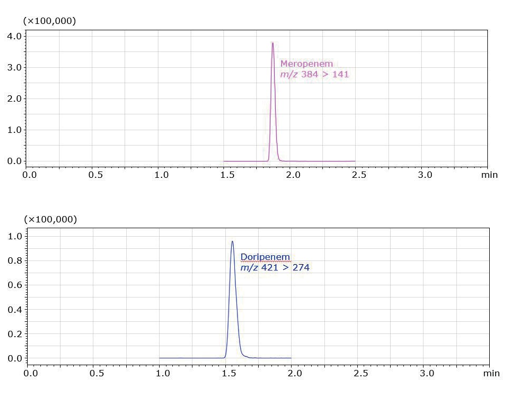
Figure 3. SRM Chromatograms of Meropenem (top) and Doripenem (bottom)
standard solutions, at a concentration of 100 ng/mL.
Results
Figure 3 shows the SRM (Selected Reaction Monitoring) chromatograms of LC-MS/MS analyses of 100 ng/mL reference solutions of meropenem and doripenem. The calibration curve generated is shown in Figure 4. Meropenem has a wide dynamic range of 1–500 ng/mL and doripenem has a dynamic range of 1–200 ng/mL. The multiple correlation coefficient (r2) is 0.997 or more over these ranges, indicating good linearity.
Since the blood concentrations of meropenem and doripenem are expected to range from 10 to 2000 ng/mL in clinical practice, we included a dilution step (dilution of meropenem 5-fold and doripenem 10-fold) prior to analysis.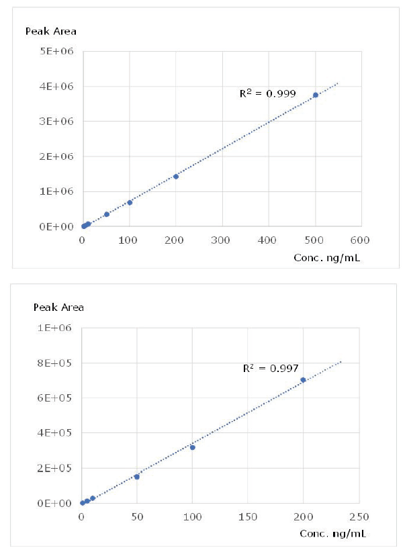
Figure 4. Calibration Curves for Meropenem (top) and Doripenem (bottom).
Confirmation of analyte recovery and matrix factors
Sample preparation is very important in samples of biological origin. ISOLUTE® ENV+ solid phase extraction columns were used to eliminate the effects of matrix components like protein, phospholipids and salts, and to to allow for improved quantitative analyses. Samples were prepared by adding meropenem or doripenem to the control plasma. Plasma concentrations were at 10.0 ng/mL . After SPE with ISOLUTE ENV+, SRM chromato- grams obtained from LC-MS/MS measurements are shown in Figure 5. Neither of the chromatograms for meropenem or doripenem showed interference by contaminants.
Next, we evaluated matrix effects using ISOLUTE ENV+ for sample clean up. Table 2 shows the recoveries and matrix factors for extraction of plasma samples spiked with meropenem or doripenem, with varying spiked concentrations of 10, 500 and 2000 ng/mL. Recovery rate was calculated by comparing the peak area value in samples spiked with with meropenem or doripenem (A) before extraction, and the peak area value in samples spiked with meropenem or doripenem after extraction (B). Matrix factors were calculated by comparing the peak area value (B) and the peak area value (S) of the standard solution in samples pre-treated with plasma alone followed by spiking with meropenem or doripenem. As a result, we were able to confirm that the matrix factor was sufficiently small at 8.5% or less. The recovery rate was also good. We were able to confirm that pretreatment with ISOLUTE ENV+ could efficiently remove the matrix effects.
Table 2. Recovery Rate and Matrix Factors (n=2) in the Spike Recovery Test.
|
|
Blood Concentrations (ng/mL) |
Recovery Rate % |
Matrix Factor % |
|
|
10 |
87.0 |
8.5 |
|
Meropenem |
500 |
85.6 |
6.7 |
|
|
2000 |
77.6 |
6.1 |
|
|
10 |
74.0 |
-7.3 |
|
Doripenem |
500 |
69.4 |
4.2 |
|
|
2000 |
67.8 |
8.2 |
* Recovery rate = [A]/[B] × 100; Matrix Factor = 1-[B]/[S] × 100.
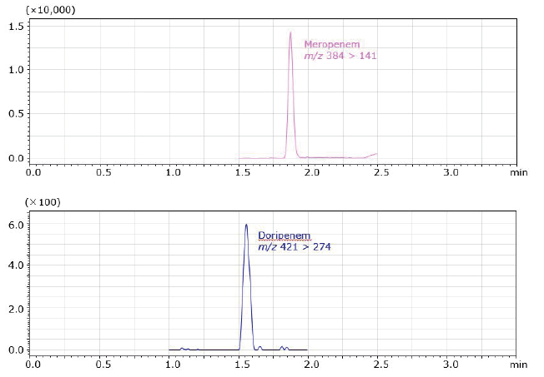
Figure 5. SRM Chromatograms after SPE with ISOLUTE® ENV+ for meropenem or doripenem, 10 ng/mL in plasma.
Ordering information
|
Part Number |
Description |
Quantity |
|---|---|---|
|
915-0002-A |
ISOLUTE® ENV+ 25 mg/1 mL |
100 |
|
915-0040-P01 |
ISOLUTE®-96 ENV+40 mg Fixed Well Plate |
1 |
|
PPM-48 |
Biotage® PRESSURE+ 48Positive Pressure Manifold |
1 |
|
121-2016 |
Biotage® VacMaster™ 20Sample Processing Manifold |
1 |
Acknowledgement
This application note was prepared in collaboration with the Pharmaceutical Department of Gunma University Hospital, Japan.
Literature number: AN947

