Jan 20, 2023 11:55:14 AM
Detecting the undetectable in flash column chromatography using wavelength focusing
By Bob Bickler

Sometimes it feels as if organic chemistry and chromatography are a mixture of art and science. Maybe its because of the necessary creativity needed to address the variety of challenges that we face almost daily. Frankly, its what I find most interesting about this world.
One of the bigger challenges facing chemists is the ability to detect and collect compounds with little or no UV absorption during flash purification. In this post I will talk about a technique that I have found to be quite useful when trying to purify mixtures containing one or more poor absorbers.
Now that most automated flash systems come with photodiode array (PDA) UV detectors, chemists have a greatly enhanced ability to detect compounds. Several options are typically available including selecting one or two specific wavelengths or detecting with all available wavelengths.
Selecting one or two specific wavelengths is fine for routine work but if you need to collect more of your product, a single wavelength may not be good enough. Since organic molecules absorb UV light over bands covering several wavelengths, selecting one or two specific lambda’s will limit the UV response compared to looking at a wavelength range.
However, even using a range of wavelengths may not improve detection unless the range is narrow. This is especially true for those organic compounds not blessed with aromaticity, conjugation, or other UV absorbing functionality.
As an example I developed a method for the separation of methyl stearate and cholesterol. Methyl stearate (Figure 1) is a fully saturated molecule with a UV maximum around 205 nm (Figure 2). Cholesterol has but a single double bond (Figure 3) and a UV maximum near 200 nm (Figure 4). My sample contained 0.425 grams of each compound in 1 mL of DCM; the injection amount was 85 mg.
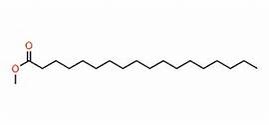
Figure 1. Methyl stearate structure. Source: Telstra.ote.cmu.edu
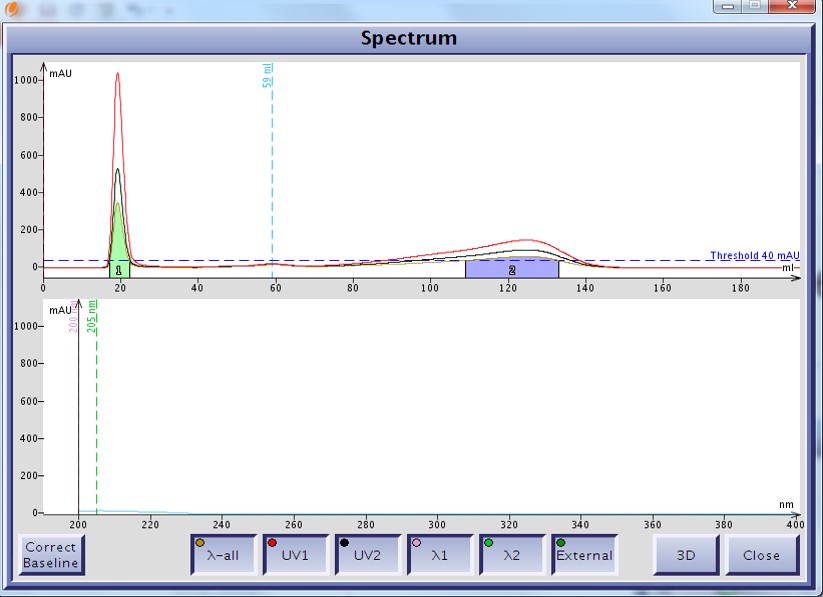
Figure 2. UV spectrum of methyl stearate as displayed on the Isolera system. The fully saturated molecule's carbonyl poorly absorbs UV with a maximum wavelength around 205 nm.
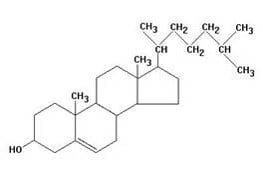
Figure 3. Cholesterol structure. Source: pherobase.com
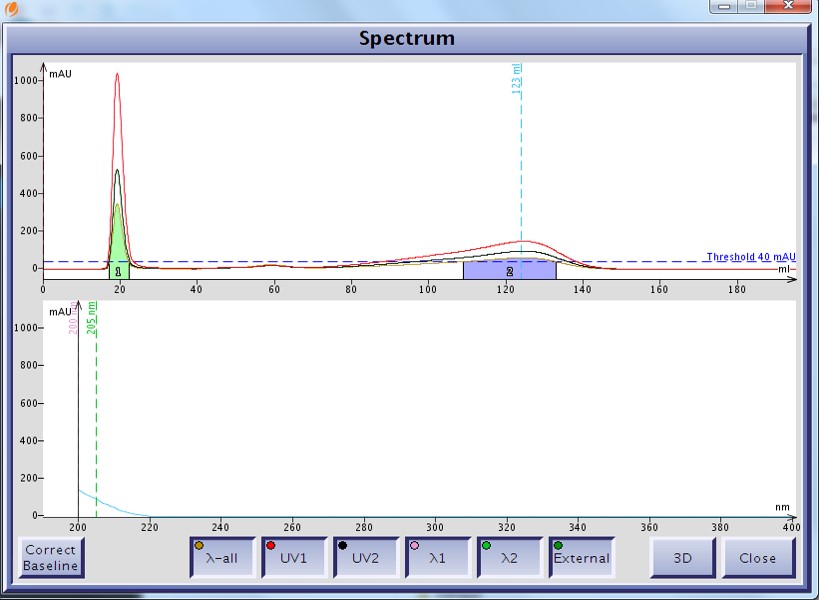
Figure 4. UV spectrum of cholesterol as displayed by the Isolera flash system shows a UV max at 200 nm but with little absorption.
The purification method was simply 100% methanol using a 12 gram Biotage® SNAP Ultra C18 cartridge at 25 mL/min. For detection I chose to select two individual wavelengths, 200 nm and 205 nm, as well as the all-wavelength capability provided by my flash system, the Biotage Isolera™ Spektra Four.
The all-wavelength capability allows the Isolera to use all of the diodes to detect whatever UV energy is absorbed at all selected wavelengths. This system’s wavelength range is 200 nm to 400 nm and any UV absorption in this range is actually summed up and the total absorption divided by the number of selected wavelengths (helps keep very strong absorption on scale). To learn more about the Isolera you can visit www.biotage.com.
For these compounds with little absorption, utilizing the entire 200-400 nm wavelength range is not effective as the total absorption is averaged across 200 wavelengths, but then again, neither is using single wavelengths of 200 nm and 205 nm, Figure 5. At 200 nm, methyl stearate is not detected and therefore not collected. Cholesterol is detected but its maximum response is under 200 mAU meaning that a lot of the compound will go to waste.
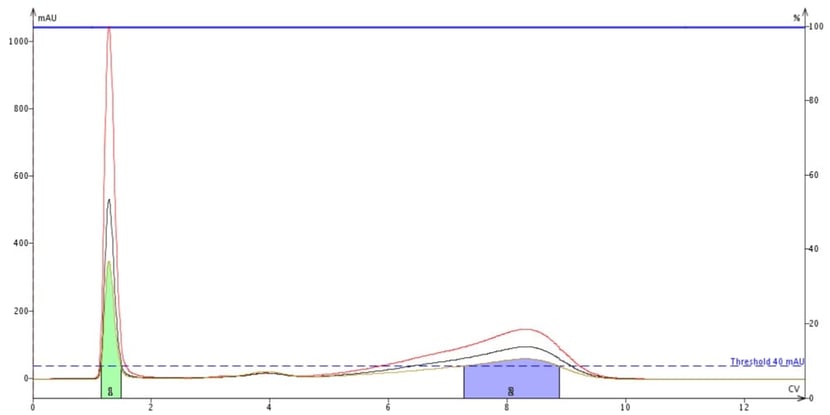
Figure 5. Flash purification of a mix of methyl stearate (peak at about 4 CV) and cholesterol (peak at about 8 CV) with detection set to 200 nm (red trace), 205 nm (black trace), and all wavelengths (200-400 nm, tan trace). The green peak is DCM, the sample dissolution solvent. Though the UV max for these compounds is 200 nm, methyl stearate is barely detectable and is not collected.
Instead, it makes sense to tailor the range of wavelengths to that of the compounds being purified. In this case, these two compounds absorb UV in a narrow band – 200 to 220 nm - but with the strongest response between 200 and 210 nm. To show the effectiveness of wavelength focusing I repeated the purification but set the all-wavelength range to 200-210 nm to enhance detection, Figure 6.
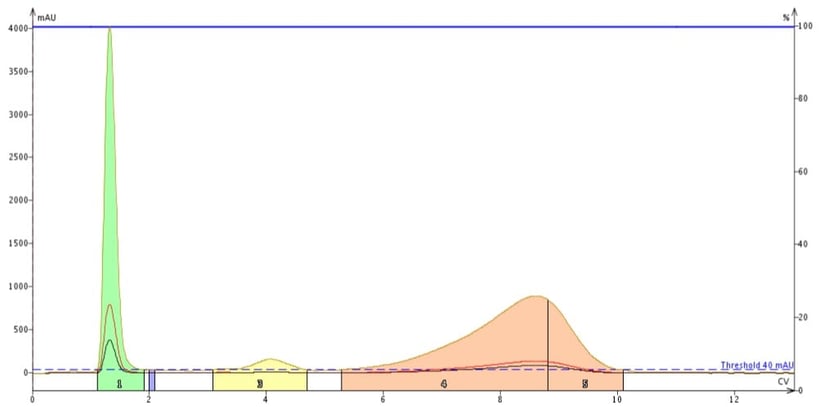
Figure 6. Impact of wavelength focusing on detection sensitivity for methyl stearate and cholesterol. Specific wavelengths of 200 nm (red) and 205 nm (black) along with a focused range between 200 and 210 nm (tan) were used for detection. The focused range provided much better sensitivity for the poor UV absorbers allowing for detection and collection of more of each compound.
As you can see using wavelength focusing greatly improves compound detection. Where methyl stearate was previously not collected, even with a single wavelength at lambda max, using the focused wavelength range of 200-210 nm sees more of the compound and it is detected and collected. The UV absorbance for cholesterol also is increased - about 5 to 6 x over a single wavelength selection (200 nm). The benefit to this wavelength focusing technique is that more compound is collected and less goes to waste.
If you think about this you will realize this is an excellent technique to detect not only these fatty compounds but for peptides and other nearly UV transparent compounds as well.
Have you tried this technique? Let me know your results.
For more about flash chromatography, check out my webinar on improving your purification.
(Thanks to idil-fibres-optiques.com for use of their focusing lense image)
Published: Jan 20, 2023 11:55:14 AM

