Aug 8, 2023 10:02:24 PM
How do weakly acidic analytes and EVOLUTE®AX work?
By Amber Cain

We know that compounds can be acidic, basic and neutral. Looking at pKas and functional groups can help us understand the best approach for SPE selection. Let’s take a look at the compound, methylmalonic acid.
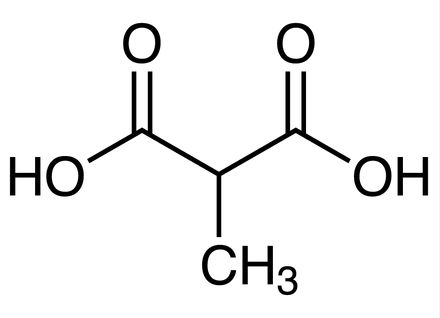
Figure 1. Structure of methylmalonic acid.
When we look at the structure we can see there is no conjugation. Without this conjugation, we will have very little potential for hydrophobic interactions. Now let’s look at the pKas of the functional groups.
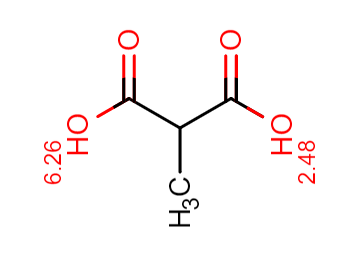
Figure 2. Methylmalonic acid pKas per Chemicalize.com.
In figure 2 we can see our most acidic functional group has a pKa of 2.48 and we don’t have any basic functional groups. Remembering some fundamentals of general chemistry, we know strong acids and strong bases are ionized over pretty much the entire usable pH range. Well, the opposite is true for weak acids and weak bases. Ionization for weak acids and bases can be controlled (effectively turned on or off) by manipulation of pH. So what constitutes strong vs weak? Let’s take a look at the sorbent chemistry selection guide in the EVOLUTE Express User Guide.
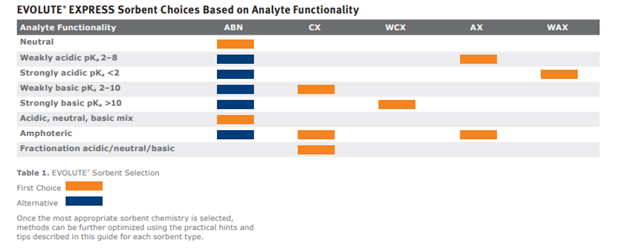
Figure 3. Looking at the analyte functionality column we can see the pKa ranges for each categorization, we will use this to help us determine the best sorbent chemistry for analysis.
The acidic pKa of 2.48 falls within the “weakly acidic” category. For a quick refresher on pH adjustment refer to the pH adjustment sample prep blog. Since we have a weakly acidic functional group, we will exploit that ion exchange potential by using the EVOLUTE®AX sorbent.
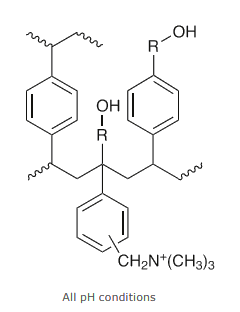
Figure 4. EVOLUTE AX Sorbent, the modified polysystrenedivinylbenzene polymer allows the sorbent to be water-wettable.
The AX sorbent has a dual non-polar (hydrophobic) and strong anion exchange functionality. The modified quaternary amine functional group is positively charged and therefore retains negatively charged analytes. We can see in Figure 4 the ionic interactions. Quaternary amines are strongly basic which tells us they are going to be 100% ionized over the entire usable pH range. We can also see that the aromatic polymer backbone of the sorbent allows for hydrophobic interactions.
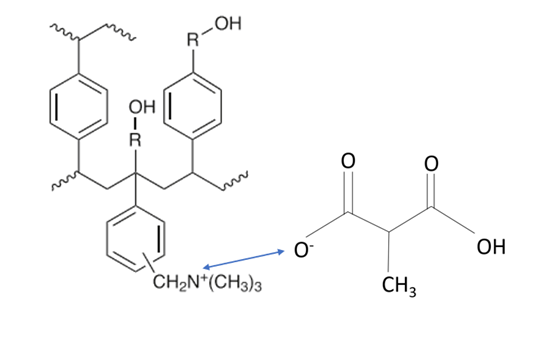
Figure 5. Hydrophobic and ionic interactions occur simultaneously between the AX sorbent and the methyl malonic acid molecule.
Now let’s see how each step occurs in the AX. In this example we are working with human serum and will use the EVOLUTE® Express AX 30 mg 96- well plate part number: 603-0030-PX01.
|
Step |
Description |
What’s happening? |
|
Sample Pre-treatment (1) |
10 µL 1 ng/µL internal standard and 100 µL human serum |
Adding the internal standard first is important as you are going to normalize to this during analysis. Typically avoid doing more than 10% volume internal standard addition. |
|
Sample Pre-treatment (2) |
290 µL HPLC grade water |
Diluting the sample will help decrease viscosity allowing it to more readily flow through the SPE sorbent. |
|
Condition (optional) |
N/A |
This is optional when using the EVOLUTE Express line due to the water-wettable functional groups (and frits). |
|
Positive Pressure |
N/A |
Positive pressure avoids variable well-to-well flow-through. Nitrogen is typically preferred to prevent oxidation and purity prevents contamination. |
|
Equilibration (optional) |
N/A |
This is optional when using the EVOLUTE Express line due to the water-wettable functional groups (and frits). |
|
Positive Pressure |
N/A |
Positive pressure avoids variable well-to-well flow-through. Nitrogen is typically preferred to prevent oxidation and purity prevents contamination. |
|
Sample Loading |
400 µL pretreated sample |
This is within the recommended load capacity. |
|
Positive Pressure |
Recommend positive pressure
|
Positive pressure avoids variable well-to-well flow-through. Nitrogen is typically preferred to prevent oxidation and purity prevents contamination. |
|
Wash 1 |
1mL HPLC water |
Remove polar interferences such as salts and small proteins. |
|
Positive Pressure |
Recommend positive pressure
|
Positive pressure avoids variable well-to-well flow-through. Nitrogen is typically preferred to prevent oxidation and purity prevents contamination. |
|
Wash 2 |
1 mL methanol |
Breaks hydrophobic interactions, releasing hydrophobic compounds such as phospholipids and lysophospholipids. |
|
Positive Pressure |
Recommend positive pressure
|
Positive pressure avoids variable well-to-well flow-through. Nitrogen is typically preferred to prevent oxidation and purity prevents contamination. |
|
Elution |
1 mL 2% formic acid in acetonitrile |
Adding the acidic buffer will neutralize the analyte, breaking the ionic interactions, and will release the analytes from the sorbent. |
|
Positive Pressure |
Recommend positive pressure
|
Positive pressure avoids variable well-to-well flow-through. Nitrogen is typically preferred to prevent oxidation and purity prevents contamination. |
|
Dry Down |
Stream of air or nitrogen |
Nitrogen is typically preferred to prevent oxidation and purity prevents contamination. |
|
Reconstitution |
100 µL 0.4% formic acid in water |
Mass spectrometry suitable solvent. |
The typical process for AX sample preparation is based on both reverse phase and ion-exchange interactions. In figure 4 we mentioned the added benefit of the polymeric sorbent. Why is this important? In traditional silica-based SPE, you had to condition and equilibrate your sorbent and frits, which were hydrophobic. With this polymeric SPE sorbent, both the sorbent and frits are water wettable and with an aqueous sample, the sorbent is able to interact with your sample without conditioning and equilibration. This also has the benefit of removing the variability of wells drying out during your analysis. This is especially beneficial if you are working with a 96-well plate and well A1 might be solvated several minutes before H12; the water-wettable capability of the EVOLUTE Express sorbent minimizes the risk of wells drying and well-to-well variability in your application results.
Published: Aug 8, 2023 10:02:24 PM


